Sandbox Reserved 426
From Proteopedia
| Line 29: | Line 29: | ||
<Structure load='3kgt' size='300' frame='true' align='right' caption='pdbcode, Insert caption here' scene='' /> <scene name='48/483883/Ligand_bindings/1'>Hydrogen Bonding and Hydrophobic interactions are shown here. </scene> As you can see, there are a total of five interactions. The Hydrogen Bonds are colored blue, and the hydrophobic interactions are colored pink. The orange color is where both hydrogen bonding and hydrophobic interactions occur at the same time. | <Structure load='3kgt' size='300' frame='true' align='right' caption='pdbcode, Insert caption here' scene='' /> <scene name='48/483883/Ligand_bindings/1'>Hydrogen Bonding and Hydrophobic interactions are shown here. </scene> As you can see, there are a total of five interactions. The Hydrogen Bonds are colored blue, and the hydrophobic interactions are colored pink. The orange color is where both hydrogen bonding and hydrophobic interactions occur at the same time. | ||
| - | <br><br><br><br><br><br><br> | + | <br> NOT FINALIZED |
| + | <br><br><br><br><br><br> | ||
Outline - Hydrogen Bonds - consist of A:LYS15(3.0) A:SER117 (3.0)/ B:LYS15(3.) B:SER117 (2.7) | Outline - Hydrogen Bonds - consist of A:LYS15(3.0) A:SER117 (3.0)/ B:LYS15(3.) B:SER117 (2.7) | ||
<br> | <br> | ||
| - | Hydrophobic interaction | + | Hydrophobic interaction LYS15 LEU17 - LEU110 ALA108 |
<br> | <br> | ||
neighboring residues which create the binding pocket / The tetramer forms a central hydrophobic pocket (T4 channel) with two binding sites for the ligand. | neighboring residues which create the binding pocket / The tetramer forms a central hydrophobic pocket (T4 channel) with two binding sites for the ligand. | ||
Revision as of 00:32, 1 April 2015
| This Sandbox is Reserved from January 19, 2016, through August 31, 2016 for use for Proteopedia Team Projects by the class Chemistry 423 Biochemistry for Chemists taught by Lynmarie K Thompson at University of Massachusetts Amherst, USA. This reservation includes Sandbox Reserved 425 through Sandbox Reserved 439. |
Contents |
Human Transthyretin (TTR) complexed with genistein
Introduction
|
1. Structure and characteristics of Transthyretin (TTR), where it can be found and its relevant concentration.
2. Function of TTR, what molecules it binds to and other responsibilities!
3. How TTR creates amorphous aggregates and/or amyloid fibrils.
4. Introduction of different amyloid diseases than can be created by the deposition of aggregates composed of variants of TTR.
Human Transthyretin (TTR) is a gene that provides instructions for the producing of a protein called transthyretin. Transthyretin is composed of identical 127-aa sandwich subunits (shown in pink) that are produced primarily in the liver.
Overall Structure
|
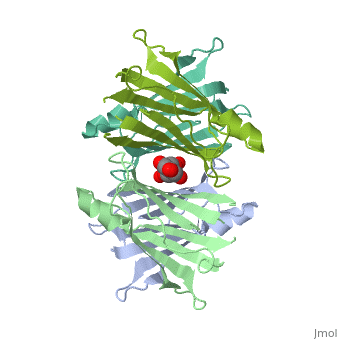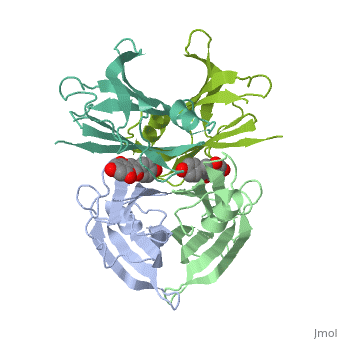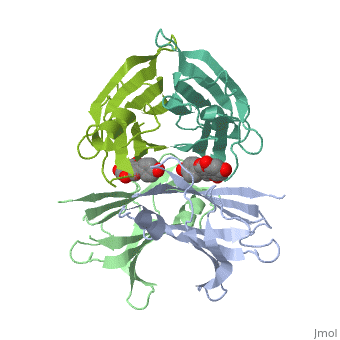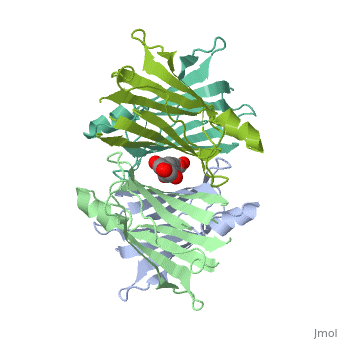
Human transthyretin (TTR) is a 55 kDa homotetramer (or more precisely, a dimer of dimers) that transports thyroxine and retinol-binding protein in the blood and cerebrospinal fluid. The monomer consists of two four-stranded β-sheets, arranged in a sandwich-like tertiary structure. The intermolecular contacts formed by the dimer–dimer interface result in the formation of a spacious channel (40 A ̊ long) running along the twofold symmetry axis of the protein. The channel is about 10 A ̊ wide at the outer rim and narrows in the centre to about 4 A ̊ . This narrowing is defined by the alignment of and on the bottom of the cleft.
Binding Interactions
|
NOT FINALIZED
Outline - Hydrogen Bonds - consist of A:LYS15(3.0) A:SER117 (3.0)/ B:LYS15(3.) B:SER117 (2.7)
Hydrophobic interaction LYS15 LEU17 - LEU110 ALA108
neighboring residues which create the binding pocket / The tetramer forms a central hydrophobic pocket (T4 channel) with two binding sites for the ligand.
The inner sheets of the dimer–dimer (AB–CD) interface – strands A, D, G and H – form two ligand-binding site cavities, which we will refer to as sites AC and BD, respectively
The side chains from residues Lys15 and Ser117, placed at the entrance and bottom of the binding sites, respectively, make important polar contacts with the genistein hydroxyl groups (Fig. 1). Furthermore, the apolar portion of residues Leu17, Leu110, Lys15 and Ala108 contribute to genistein binding through hydrophobic contacts, as suggested by the ligand-binding analysis
Additional Features
|
-insert possible new starting green scene
-the binding of substrate to Transthyretin requires four TTR proteins to be bound to each other simultaneously.
-four-protein unit (tetramer)
-binding and transport of thyroxine and retinol requires tetramer
-retinol requires retinol binding protein
-
Quiz Question 1
|
A. An increase in binding affinity and increase in hydrophobic interactions.
B. A decrease or even complete loss of binding affinity.
C. No change in affinity, both polar and nonpolar interactions bind retinol-binding protein and transthyretin.
Answer:B
Quiz Question 2
|
See Also
Credits
Introduction - name of team member
Overall Structure - name of team member
Drug Binding Site - name of team member
Additional Features - name of team member
Quiz Question 1 - name of team member
Quiz Question 2 - name of team member