This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1063
From Proteopedia
| Line 1: | Line 1: | ||
== Introduction == | == Introduction == | ||
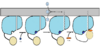
| - | ''<scene name='69/694230/Fadd13_subunits/3'>FadD13</scene>'' in ''Mycobacterium Tuberculosis'' is an ACSVL (Acyl-CoA synthetases very long) peripheral membrane protein. ACS proteins activate lipids and fatty acids before going into metabolic pathways. FadD13 is soluble unlike other ACSVL proteins. FadD13 contains a hydrophobic tunnel for fatty acids to bind to, as well as an arginine rich lid loop that binds to the cell membrane. The binding of ATP causes structural changes promoting the binding of the hydrophobic substrates. Formation of an acyl-adenylate intermediate induces a 140 degree rotation of the small domain and binding of CoA for production of the final product, a fatty acyl-CoA thioester. | + | ''<scene name='69/694230/Fadd13_subunits/3'>FadD13</scene>'' in ''Mycobacterium Tuberculosis'' is an ACSVL (Acyl-CoA synthetases very long) peripheral membrane protein. ACS proteins activate lipids and fatty acids before going into metabolic pathways. FadD13 is soluble unlike other ACSVL proteins. FadD13 contains a hydrophobic tunnel for fatty acids to bind to, as well as an arginine rich lid loop that binds to the cell membrane. The binding of ATP causes structural changes promoting the binding of the hydrophobic substrates. Formation of an acyl-adenylate intermediate induces a 140 degree rotation of the small domain and binding of CoA for production of the final product, a fatty acyl-CoA thioester. Below is the general mechanism for ACS proteins. |
[[Image:FadD13 steps.jpg|100 px|thumb|left|Mechanism]] | [[Image:FadD13 steps.jpg|100 px|thumb|left|Mechanism]] | ||
| Line 13: | Line 13: | ||
== Function == | == Function == | ||
| - | The FadD13 enzyme functions to activate lipids before going into metabolic pathways. This is done by ATP/AMP binding to the <scene name='69/694230/Atp_and_amp_binding_region/4'>ATP and AMP binding region</scene>. Once ATP/AMP is bound the long lipid chain up to 26 carbons may bind in the hydrophobic portion of the enzyme. Upon binding of the substrate, the C terminal swings up to close off the tunnel. From There CoA can bind to produce the final product, an acyl-CoA Thioester. The lipid can now move transversely throughout the membrane and throughout the rest of the cell. | + | The FadD13 enzyme functions to activate lipids before going into metabolic pathways. This is done by ATP/AMP binding to the <scene name='69/694230/Atp_and_amp_binding_region/4'>ATP and AMP binding region</scene>. Once ATP/AMP is bound the long lipid chain up to 26 carbons may bind in the hydrophobic portion of the enzyme. Upon binding of the substrate, the C terminal swings up to close off the tunnel. From There CoA can bind to produce the final product, an acyl-CoA Thioester. The lipid can now move transversely throughout the membrane and throughout the rest of the cell. Below is the proposed mechanism for ACSVL proteins. |
[[Image:Proposed Mechanism.png|100 px|thumb|left|Proposed Mechanism]] | [[Image:Proposed Mechanism.png|100 px|thumb|left|Proposed Mechanism]] | ||
Revision as of 12:44, 7 April 2015
Introduction
in Mycobacterium Tuberculosis is an ACSVL (Acyl-CoA synthetases very long) peripheral membrane protein. ACS proteins activate lipids and fatty acids before going into metabolic pathways. FadD13 is soluble unlike other ACSVL proteins. FadD13 contains a hydrophobic tunnel for fatty acids to bind to, as well as an arginine rich lid loop that binds to the cell membrane. The binding of ATP causes structural changes promoting the binding of the hydrophobic substrates. Formation of an acyl-adenylate intermediate induces a 140 degree rotation of the small domain and binding of CoA for production of the final product, a fatty acyl-CoA thioester. Below is the general mechanism for ACS proteins.
| |||||||||||
References
Andersson, C.S., Lundgren, C.A.K., Magnusdottir, A., Ge, C., Weislander, A., Molina, D., Hogbom, M. (2012)The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: structural Basis for Housing Lipid Substrates longer than the Enzyme. Cell Press,1062-1070