We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1063
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
== Function == | == Function == | ||
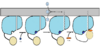
| - | The FadD13 enzyme functions to activate lipids before going into metabolic pathways. This is done by ATP/AMP binding to the <scene name='69/694230/Atp_and_amp_binding_region/4'>ATP and AMP binding region</scene>. Once ATP/AMP is bound the long lipid chain up to 26 carbons may bind in the hydrophobic portion of the enzyme. Upon binding of the substrate, the C terminal swings up to close off the tunnel. From There CoA can bind to produce the final product, an acyl-CoA Thioester. The lipid can now move transversely throughout the membrane and throughout the rest of the cell. Below is the proposed mechanism for ACSVL proteins. | + | The FadD13 enzyme functions to activate lipids before going into metabolic pathways. This is done by ATP/AMP binding to the <scene name='69/694230/Atp_and_amp_binding_region/4'>ATP and AMP binding region</scene>. Once ATP/AMP is bound the long lipid chain up to 26 carbons may bind in the hydrophobic portion of the enzyme. Upon binding of the substrate, the C terminal swings up to close off the tunnel. From There CoA can bind to produce the final product, an acyl-CoA Thioester. The lipid can now move transversely throughout the membrane and throughout the rest of the cell. Below is the proposed mechanism for ACSVL proteins<ref> [Andersson, C.S., Lundgren, C.A.K., Magnusdottir, A., Ge, C., Weislander, A., Molina, D., Hogbom, M. (2012)The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: structural Basis for Housing lipid Substrates longer than the Enzyme. Cell Press,1062-1070] </ref>. |
[[Image:Proposed Mechanism.png|100 px|thumb|left|Proposed Mechanism]] | [[Image:Proposed Mechanism.png|100 px|thumb|left|Proposed Mechanism]] | ||
</StructureSection> | </StructureSection> | ||
| - | == References == | + | == References == |
| - | * Andersson, C.S., Lundgren, C.A.K., Magnusdottir, A., Ge, C., Weislander, A., Molina, D., Hogbom, M. (2012)The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: structural Basis for Housing lipid Substrates longer than the Enzyme. Cell Press,1062-1070 | + | {{reflist}} |
| + | * Andersson, C.S., Lundgren, C.A.K., Magnusdottir, A., Ge, C., Weislander, A., Molina, D., Hogbom, M. (2012)The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: structural Basis for Housing lipid Substrates longer than the Enzyme. Cell Press,1062-1070 </ref> | ||
* [http://www.proteopedia.org/wiki/index.php/3r44 3R44] | * [http://www.proteopedia.org/wiki/index.php/3r44 3R44] | ||
Revision as of 13:42, 7 April 2015
FadD13
| |||||||||||
References
- ↑ [Andersson, C.S., Lundgren, C.A.K., Magnusdottir, A., Ge, C., Weislander, A., Molina, D., Hogbom, M. (2012)The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: structural Basis for Housing lipid Substrates longer than the Enzyme. Cell Press,1062-1070]
- Andersson, C.S., Lundgren, C.A.K., Magnusdottir, A., Ge, C., Weislander, A., Molina, D., Hogbom, M. (2012)The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: structural Basis for Housing lipid Substrates longer than the Enzyme. Cell Press,1062-1070 </ref>