User:Braden Sciarra/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
==Mechanism== | ==Mechanism== | ||
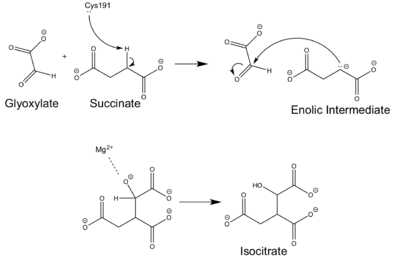
[[Image:ICL Mechanism.png|400 px|right|thumb|Figure 4: Chemical Mechanism of Isocitrate Lyase]] | [[Image:ICL Mechanism.png|400 px|right|thumb|Figure 4: Chemical Mechanism of Isocitrate Lyase]] | ||
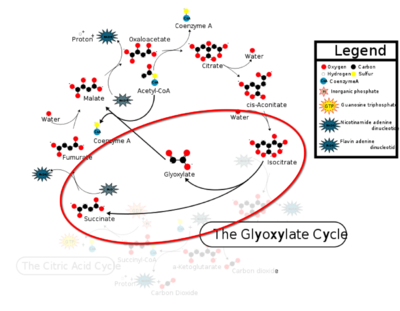
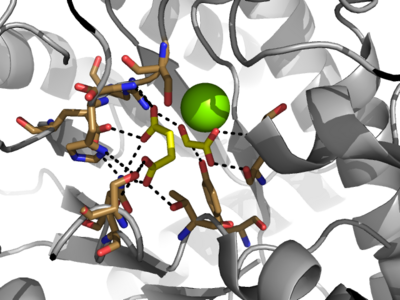
| - | Isocitrate lyase catalyzes a reversible aldol condensation, converting isocitrate to glyoxylate and succinate via the breaking of a C-C bond<ref>DOI: 10.1021/ja00187a053</ref>. Within the active site of ICL the HIS193 residue deprotonates the CYS191 residue of the active site in order to increase its basicity<ref name="ICL">PMID:10932251</ref>. The Cys 191 residue then deprotonates the alpha carbon adjacent to one of the carbonyl groups of succinate, thus forming the enolic intermediate. The negatively charged alpha carbon atom of the enolic intermediate acts as a nucleophile that attacks the carbonyl carbon of the aldehyde of glyoxylate. The nucleophilic attack will place a negative charge on the oxygen atom oxygen at the former carbonyl oxygen of the aldehyde, which will be stabilized by positive charges of the Mg2+ ion, ARG228 and HIS180<ref name="ICL">PMID:10932251</ref>. The protonation of this species will yield the final product. It is important to note that this reaction is entirely reversible; the breakdown of isocitrate into glyoxylate and succinate occurs using a similar mechanism. | + | Isocitrate lyase catalyzes a reversible aldol condensation, converting isocitrate to glyoxylate and succinate via the breaking of a C-C bond<ref>Masamune et al. Bio-Claisen condensation catalyzed by thiolase from Zoogloea ramigera. Active site cysteine residues. "Journal of the American Chemical Society" 1989 111: 1879-1881 (1989). DOI: 10.1021/ja00187a053</ref>. Within the active site of ICL the HIS193 residue deprotonates the CYS191 residue of the active site in order to increase its basicity<ref name="ICL">PMID:10932251</ref>. The Cys 191 residue then deprotonates the alpha carbon adjacent to one of the carbonyl groups of succinate, thus forming the enolic intermediate. The negatively charged alpha carbon atom of the enolic intermediate acts as a nucleophile that attacks the carbonyl carbon of the aldehyde of glyoxylate. The nucleophilic attack will place a negative charge on the oxygen atom oxygen at the former carbonyl oxygen of the aldehyde, which will be stabilized by positive charges of the Mg2+ ion, ARG228 and HIS180<ref name="ICL">PMID:10932251</ref>. The protonation of this species will yield the final product. It is important to note that this reaction is entirely reversible; the breakdown of isocitrate into glyoxylate and succinate occurs using a similar mechanism. |
==Elucidation of ICL Structure Using Inhibitors== | ==Elucidation of ICL Structure Using Inhibitors== | ||
Revision as of 00:18, 8 April 2015
Isocitrate Lyase from Mycobacterium Tuberculosis
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Sharma V, Sharma S, Hoener zu Bentrup K, McKinney JD, Russell DG, Jacobs WR Jr, Sacchettini JC. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat Struct Biol. 2000 Aug;7(8):663-8. PMID:10932251 doi:10.1038/77964
- ↑ Masamune et al. Bio-Claisen condensation catalyzed by thiolase from Zoogloea ramigera. Active site cysteine residues. "Journal of the American Chemical Society" 1989 111: 1879-1881 (1989). DOI: 10.1021/ja00187a053