User:Braden Sciarra/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='1F8I' size='340' side='right' caption='Isocitrate Lyase from ''Mycobacterium tuberculosis''' scene=''> | <StructureSection load='1F8I' size='340' side='right' caption='Isocitrate Lyase from ''Mycobacterium tuberculosis''' scene=''> | ||
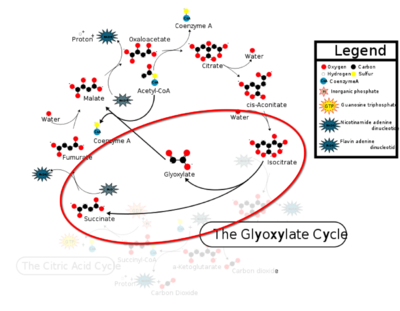
[[Image:CAC.png|400 px|right|thumb|Figure 1: ICL mediated glyoxylate shunt pathway of the Citric Acid Cycle]] | [[Image:CAC.png|400 px|right|thumb|Figure 1: ICL mediated glyoxylate shunt pathway of the Citric Acid Cycle]] | ||
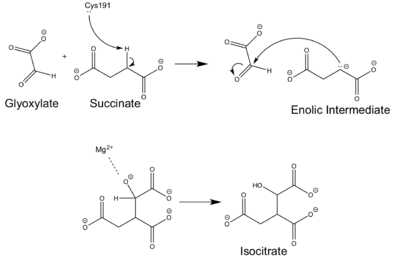
| - | [http://en.wikipedia.org/wiki/Isocitrate_lyase Isocitrate Lyase] (ICL) is a metabolic enzyme that converts the metabolite isocitrate into glyoxylate and succinate. ICL is a homotetramer with each monomer being composed of 14 alpha helices, 14 beta sheets, and a magnesium ion cofactor. ICL has shown clinical relevance in the disease state [http://en.wikipedia.org/wiki/Tuberculosis Tuberculosis] where it is responsible for the persistence of Mycobacterium tuberculosis during the chronic stage of infection. This survival strategy mediated by ICL is characterized by a metabolic shortcut within the [http://en.wikipedia.org/wiki/Citric_acid_cycle Citric Acid Cycle]. ICL creates this shunt pathway by converting isocitrate to succinate and glyoxylate, diverting acetyl-CoA from the beta-oxidation of fatty acids<ref name="ICL">PMID:10932251</ref><ref name="ICL2"/>. | + | [http://en.wikipedia.org/wiki/Isocitrate_lyase Isocitrate Lyase] (ICL) is a metabolic enzyme that converts the metabolite isocitrate into glyoxylate and succinate. ICL is a homotetramer with each monomer being composed of 14 alpha helices, 14 beta sheets, and a magnesium ion cofactor. ICL has shown clinical relevance in the disease state [http://en.wikipedia.org/wiki/Tuberculosis Tuberculosis] where it is responsible for the persistence of Mycobacterium tuberculosis during the chronic stage of infection. This survival strategy mediated by ICL is characterized by a metabolic shortcut within the [http://en.wikipedia.org/wiki/Citric_acid_cycle Citric Acid Cycle]. ICL creates this shunt pathway by converting isocitrate to succinate and glyoxylate, diverting acetyl-CoA from the beta-oxidation of fatty acids<ref name="ICL">PMID:10932251</ref><ref name="ICL2">PMID2696959</ref>. |
== Structure == | == Structure == | ||
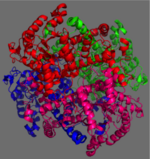
[[Image:homotetramer.png|150 px|left|thumb|Figure 2: C2 Symmetry of the homotetramer isocitrate lyase]] | [[Image:homotetramer.png|150 px|left|thumb|Figure 2: C2 Symmetry of the homotetramer isocitrate lyase]] | ||
| - | The ICL homotetramer possesses C2 symmetry, with an axis of rotation at x-axis, y-axis, and z-axis of the enzyme. Two individual subunits off ICL are held together by a characteristic <scene name='69/697526/Helix_swapping/3'>Helix Swapping</scene> between three alpha helices formed by residues 370-384, 349-367, and 399-409 on neighboring monomers<ref name="ICL">PMID:10932251</ref>. The interlocking mechanism created by these helices provides additional strength to hold the two monomeric subunits together, allowing ICL to essentially be composed of two dimerized subunits<ref name="ICL2" | + | The ICL homotetramer possesses C2 symmetry, with an axis of rotation at x-axis, y-axis, and z-axis of the enzyme. Two individual subunits off ICL are held together by a characteristic <scene name='69/697526/Helix_swapping/3'>Helix Swapping</scene> between three alpha helices formed by residues 370-384, 349-367, and 399-409 on neighboring monomers<ref name="ICL">PMID:10932251</ref>. The interlocking mechanism created by these helices provides additional strength to hold the two monomeric subunits together, allowing ICL to essentially be composed of two dimerized subunits<ref name="ICL2"/>. This interaction will bury approximately 18% of the surface of each subunit, and will help to shield the interior binding site from hydration. |
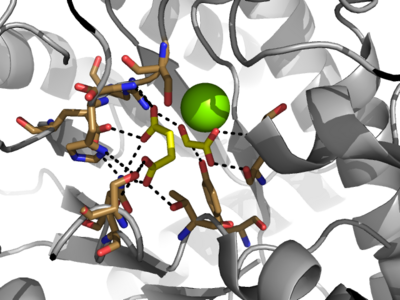
== Active Site == | == Active Site == | ||
Revision as of 00:36, 8 April 2015
Isocitrate Lyase from Mycobacterium Tuberculosis
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Sharma V, Sharma S, Hoener zu Bentrup K, McKinney JD, Russell DG, Jacobs WR Jr, Sacchettini JC. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat Struct Biol. 2000 Aug;7(8):663-8. PMID:10932251 doi:10.1038/77964
- ↑ 2.0 2.1 2.2 . PMID:216315890657
- ↑ 3.0 3.1 3.2 3.3 Masamune et al. Bio-Claisen condensation catalyzed by thiolase from Zoogloea ramigera. Active site cysteine residues. "Journal of the American Chemical Society" 111: 1879-1881 (1989). DOI: 10.1021/ja00187a053