We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1073
From Proteopedia
(Difference between revisions)
| Line 37: | Line 37: | ||
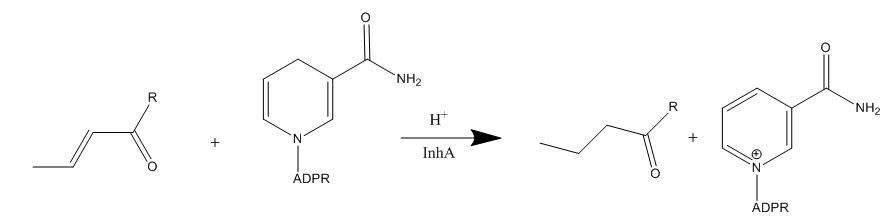
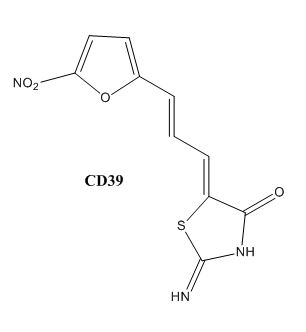
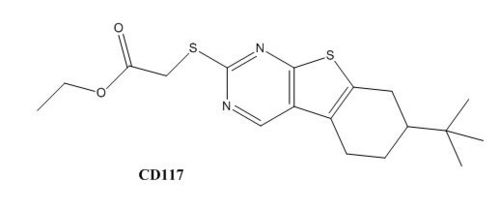
Drug resistance of ''M. tuberculosis'' has become a huge problem for the development of antibiotics. A drug screen of potential inhibitors of InhA (300 compounds), were composed of inhibitors of the [http://en.wikipedia.org/wiki/Plasmodium_falciparum ''Plasmodium falciparum''] enoyl-reductase, against ''M. tuberculosis''. The enoyl reductases of both bacteria have limited similarities, however two compounds, CD39 and CD117 had activity against drug-susceptible ''M. tuberculosis''. More importantly, both compounds had activity against drug-resistant and multi-drug resistant TB. Treatment of the bacterium with the compounds resulted in the inhibition of mycolic acid and long-chain fatty acid biosynthesis, indicating that these compounds act against enzymes of both the FAS-I and FAS-II system. The benefit of having the compounds have multiple targets is the reduced development of drug resistance, which is the disadvantage of isoniazid. The essential chemical groups that lead to the antimycobacterial properties of the compounds include a [http://en.wikipedia.org/wiki/Thioacetic_acid thioacetate] group, and a [http://en.wikipedia.org/wiki/Butyl t-butyl] group. | Drug resistance of ''M. tuberculosis'' has become a huge problem for the development of antibiotics. A drug screen of potential inhibitors of InhA (300 compounds), were composed of inhibitors of the [http://en.wikipedia.org/wiki/Plasmodium_falciparum ''Plasmodium falciparum''] enoyl-reductase, against ''M. tuberculosis''. The enoyl reductases of both bacteria have limited similarities, however two compounds, CD39 and CD117 had activity against drug-susceptible ''M. tuberculosis''. More importantly, both compounds had activity against drug-resistant and multi-drug resistant TB. Treatment of the bacterium with the compounds resulted in the inhibition of mycolic acid and long-chain fatty acid biosynthesis, indicating that these compounds act against enzymes of both the FAS-I and FAS-II system. The benefit of having the compounds have multiple targets is the reduced development of drug resistance, which is the disadvantage of isoniazid. The essential chemical groups that lead to the antimycobacterial properties of the compounds include a [http://en.wikipedia.org/wiki/Thioacetic_acid thioacetate] group, and a [http://en.wikipedia.org/wiki/Butyl t-butyl] group. | ||
| - | [[Image:CD39.JPG|thumb|500px| | + | [[Image:CD39.JPG|thumb|500px|center|Figure 2. CD39 Structure]] [[Image:CD117.JPG|thumb|500 px|center|Figure 3. CD117 Structure]] |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 22:00, 8 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Enoyl-ACP Reductase InhA
| |||||||||||