Introduction
The Enoyl-ACP Reductase InhA, from Mycobacterium tuberculosis, catalyzes the NADH-dependent reduction of long-chain trans-2-enoyl-ACP fatty acids in the type II fatty acid biosynthesis pathway of M. tuberculosis. InhA is a member of the short chain dehydrogenase/reductase (SDR) family of enzymes. InhA is the only enoyl-ACP reductase found in tuberculosis, making the enzyme a potential drug target [1].
FAS-II System
Mycolic acids are very long-chain fatty acids (C60 -C90) that are essential components of the mycobacterial cell wall. Mycolic acids are synthesized by at least two known elongation systems, type I and type II fatty acid synthases (FAS-I and FAS-II) [2]. The FAS-II system prefers C16 as a starting substrate and can extend up to C56 [3]. The FAS-II system utilizes the products from the FAS-I system as primers to extend the chain lengths further. The products of the FAS-II system are the precursors of mycolic acids. Elongation by the FAS-II system occurs by a condensation reactionof acetyl and malonyl substrates, which is achieved in three steps. Step 1 involves transfer of the acyl primer, step 2 involves decarboxylation of the substrate to yield a carbanion, and step 3 involves nucleophilic attack of the carbanion to yield the elongated product [2].
Mechanism of Action
The InhA gene encodes for the InhA protein. InhA catalyzes the NADH-dependent reduction of the trans double bond between positions C2-C3 of fatty acyl substrates. InhA prefers fatty acyl substrates of C16 or longer, which is consistent of the protein being a member of the FAS-II system. The longer chain length specificity of InhA distinguishes the enzyme from other enoyl-ACP reductase analogues.
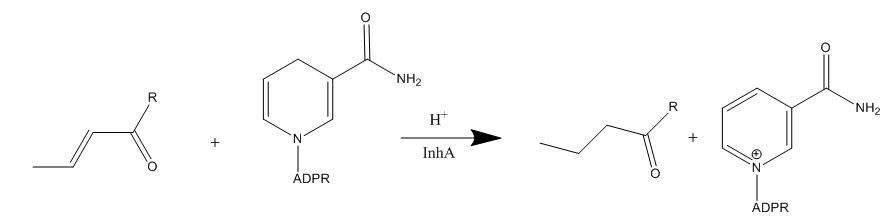
Figure 1. Mechanism of InhA protein
General Structural Information
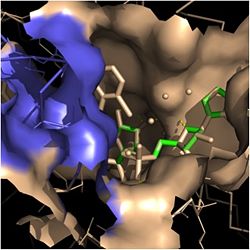
Figure 2. Fatty Acyl Binding Crevice (substrate binding loop in purple; substrates pictured inside the crevice)
Crystal structures of InhA reveal a (each subunit featured with a different color) in aqueous solution with separate ligand binding sites in each subunit [4]. Each subunit is composed of 289 residues and features a typical Rossmann fold containing a single NADH binding site. The of InhA is made up of several alpha helices (pink), beta sheets (gold), and beta turns (white) [1]. This enzyme also features a fatty acyl binding crevice that accommodates the long-chain fatty acyl substrate needed to synthesize mycolic acid precursors. The of the InhA form one side of the fatty acyl binding crevice, referred to as the (residues 196-219). One side of this crevice is open and exposed to solvent, which allows the substrates to access the binding pocket of this enzyme. The size of the substrate binding loop is a primary determinant of the ability of InhA to select for fatty acyl chains longer than 16 carbons to successfully produce mycolic acid precursors [4].
Ligands
The two ligands that play a role in the function of InhA are NADH and the long-chain fatty acyl substrate. NADH, or nicotinamide adenine dinucleotide, is a cofactor found in all living cells. It is especially important in the function of InhA because it helps position the fatty acyl substrate within the fatty acyl binding crevice, thereby improving the overall activity of InhA. Once properly positioned, the fatty acyl substrates are reduced at the trans double bond between C2 and C3, forming mycolic acid precursors that eventually compose the cell wall of mycobacteria [4].
Fatty Acyl Binding Crevice
Within the fatty acyl binding crevice, the NADH substrate sits on the top shelf of the Rossmann fold, and the fatty acyl substrate sits on top of the NADH substrate. Due to its position within the crevice, the
trans double bond of the fatty acyl substrate is found near the closed end of the crevice and is located directly adjacent to the nicotinamide ring of
NADH.
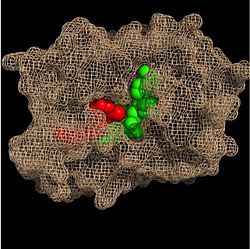
Figure 3. Substrate Binding Pocket (NADH in green; fatty acyl substrate in red)
These two molecules interact with each other via hydrogen bonding. The first hydrogen bond occurs between the phosphate oxygen of NADH and the amide nitrogen of the N-acetylcysteamine portion of the fatty acyl substrate. The second hydrogen bond occurs between the 2'-hydroxyl of the nicotinamide ribose of NADH and the thioester carbonyl oxygen of the fatty acyl substrate
[4].
Substrate Binding Loop Flexibility
In addition to the hydrogen bonding that occurs between the NADH and fatty acyl substrate within the crevice, each of these molecules is also held in place within the crevice through interactions with the side chains of surrounding (purple) residues. The majority of these residues anchoring the substrates are found within the substrate binding loop itself, including Ala-198, Met-199, Ala-201, Ile-202, Leu-207, Ile-215, and Leu-218. Additional hydrophobic amino acids that are not a part of the substrate binding loop also play a role in positioning and stabilizing the fatty acyl chain in the crevice. Studies have found that the fatty acyl substrate adopts a u-shaped conformation to facilitate binding. As the fatty acyl substrate binds in the crevice, the substrate binding loop shifts outward toward the solvent, and the [[Tyr-158 #===Importance of Tyr-158===]] residue is rotated to facilitate the binding of fatty acyl chains of 16 carbons or greater. During this conformational change upon substrate binding, no hydrogen bonds are broken, which supports the flexibility of the substrate binding loop. It is likely that this flexibility of the substrate binding loop provides increased freedom for the active site to accept fatty acyl substrates of varying carbon chain lengths [4].
Importance of Tyr-158
One example of a hydrophobic amino acid that is not a part of the substrate binding loop yet interacts with the fatty acyl substrate is
Tyr-158.
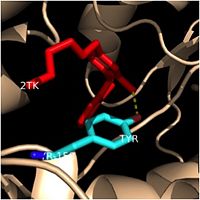
Figure 4. Tyr-158 (light blue) hydrogen bonding to the fatty acyl substrate, 2TK (red)
This amino acid is conserved in other enoyl-ACP reductases in both bacteria and plants, so it likely plays an essential role in the function of these specific enzymes. Studies have shown that Tyr-158 forms the only direct hydrogen bond that exists between the InhA protein and the fatty acyl substrate. This hydrogen bond occurs between the hydroxyl oxygen on the side chain of Tyr-158 and the thioester carbonyl oxygen of the fatty acyl substrate. Consequently, the Tyr-158 residue acts to stabilize the enolate intermediate that forms during the hydride transfer reaction
[4].
Catalytic Triad
Structural studies have shown that InhA possesses a , composed of Phe-149, Tyr-158, and Lys-165. This catalytic triad of InhA is analogous to the classic Ser-Tyr-Lys catalytic triad present in all members of the SDR (Short-chain Dehydrogenase Reductase) family [4]. In the dehydrogenases, the first catalytic residue is usually Ser or Thr, while in the enoyl reuctases (InhA), the first catalytic residue is usually Phe or Tyr [1]. According to crystallographic data, the likely role of Tyr-158 is to stabilize the enolate intermediate that forms during the hydride transfer reaction [4]. Previous structural and kinetic studies have confirmed that the side chain of the Lys-165 residue in InhA functions similarly to the catalytic Lys residues in other SDR enzymes. The catalytic Lys-165 residue in InhA interacts with the 2' or 3'-hydroxyls of the nicotinamide ring of NADH to hold this cofactor in place within the fatty acyl binding crevice [4]. Finally, the exact role of the catalytic Phe-149 residue in InhA is less well known. Recent Raman spectroscopy studies have supported that the catalytic Phe-149 residue plays an essential role in orienting the NADH cofactor correctly to lower the energy of the transition state and promoting the hydride transfer reaction [1]. Altogether, the residues in the catalytic triad of InhA play critical roles in properly orienting NADH and the fatty acyl substrate to promote the hydride transfer reaction required to elongate the fatty acyl chains and produce mycolic acid precursors.
Clinical Applications
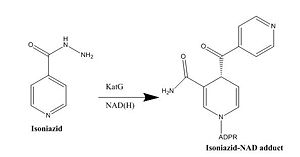
Figure 5. Isoniazid Mechanism of Action
Isoniazid
Isoniazid is a first-line antibiotic that has been used to treat tuberculosis infections for over 50 years. Isoniazid is known to inhibit mycolic acid biosnthesis, which is the function of InhA. The activated form of isoniazid is covalently attached to the nicotinamide ring of NADH. However, Isoniazid is still not an ideal antibiotic because many drug-resistant strains of tuberculosis have shown resistance to this inhibitor. Specifically, the mutation Ser94 to Ala of InhA was sufficient enough to have isoniazid resistance [4].
Other Inhibitors
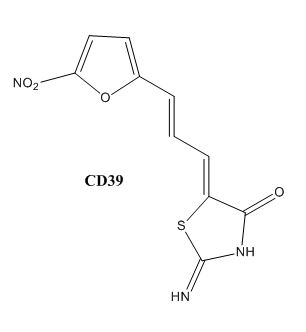
Drug resistance of M. tuberculosis has become a huge problem for the development of antibiotics. A drug screen of potential inhibitors of InhA (300 compounds), were composed of inhibitors of the Plasmodium falciparum enoyl-reductase, against M. tuberculosis. The enoyl reductases of both bacteria have limited similarities, however two compounds, CD39 and CD117 had activity against drug-susceptible M. tuberculosis. More importantly, both compounds had activity against drug-resistant and multi-drug resistant TB. Treatment of the bacterium with the compounds resulted in the inhibition of mycolic acid and long-chain fatty acid biosynthesis, indicating that these compounds act against enzymes of both the FAS-I and FAS-II system. The benefit of having the compounds have multiple targets is the reduced development of drug resistance, which is the disadvantage of isoniazid. The essential chemical groups that lead to the antimycobacterial properties of the compounds include a thioacetate group, and a t-butyl group.
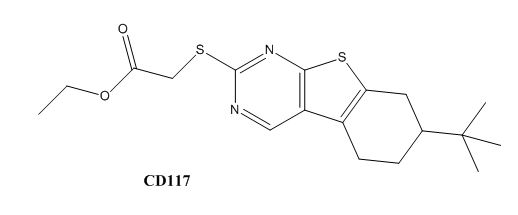
Figure 7. CD117 Structure