We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1072
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
''Mycobacterium Tuberculosis'' Catalase Peroxidase (''mt''CP) is a [http://en.wikipedia.org/wiki/Protein_dimer homodimer] with each monomer consisting of two [http://en.wikipedia.org/wiki/Protein_domain domains]. The overall structure is stabilized by 703 water molecules. | ''Mycobacterium Tuberculosis'' Catalase Peroxidase (''mt''CP) is a [http://en.wikipedia.org/wiki/Protein_dimer homodimer] with each monomer consisting of two [http://en.wikipedia.org/wiki/Protein_domain domains]. The overall structure is stabilized by 703 water molecules. | ||
| - | blue - n terminus hook | ||
| - | |||
| - | magenta - n terminus | ||
| - | |||
| - | light pink - c terminus | ||
===Monomer Structure=== | ===Monomer Structure=== | ||
| Line 25: | Line 20: | ||
===Active Site=== | ===Active Site=== | ||
| - | In ''hm''CP, which share 55% and 69% identity with ''mt''CP, the heme is buried inside ''Hm''CP-N, and substrate access to the active site is thorugh a narrow channel that prevents access of a large substrate (3). | + | |
| + | |||
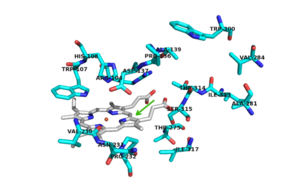
| + | [[Image:INH.png|300 px|left|thumb|Figure Legend]] | ||
| + | |||
| + | There are 6 conserved key active site residues that suround the <scene name='69/694238/Heme/2'>heme</scene>. These <scene name='69/694238/Active_site/2'>active site</scene> residues are Arg 104, Trp 107, His 108, His 270, Asp 381. | ||
| + | |||
| + | In ''hm''CP, which share 55% and 69% identity with ''mt''CP, the heme is buried inside ''Hm''CP-N, and substrate access to the active site is thorugh a narrow channel that prevents access of a large substrate (3). The location of the binding site for [http://en.wikipedia.org/wiki/Isoniazid isoniazid (INH)] is located near the ''δ meso'' heme edge, about 3.8 Å away from the heme iron. This binding site is found within what is considered to be the usual substrate access channel of peroxidases. The reaction between INH and the enzyme must occur from interaction in a binding site intended for the natural substrate (A2). Asp 137 plays a key role in the activation and binding of INH. Asp 137 creates energetically favorable interactions due to its ability to make hydrogen-bond interactions between its carboxylic acid side chain and the pyridinyl N1 of INH. | ||
==Catalase Peroxidases== | ==Catalase Peroxidases== | ||
Revision as of 01:29, 9 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
| |||||||||||