Sandbox Reserved 1074
From Proteopedia
| Line 22: | Line 22: | ||
== '''General Structural Information''' == | == '''General Structural Information''' == | ||
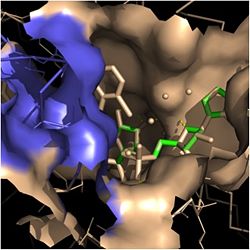
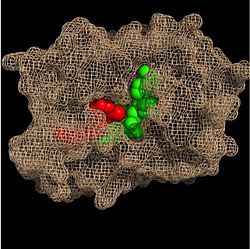
[[Image:Fatty Acyl Binding Crevice.jpg|thumb|250px|left|Figure 2. Fatty Acyl Binding Crevice (substrate binding loop in purple; substrates pictured inside the crevice)]] | [[Image:Fatty Acyl Binding Crevice.jpg|thumb|250px|left|Figure 2. Fatty Acyl Binding Crevice (substrate binding loop in purple; substrates pictured inside the crevice)]] | ||
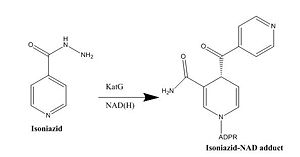
| - | Crystal structures of InhA reveal a <scene name='69/694241/Homotetramer_subunits_labeled/1'>homotetramer</scene> (each subunit featured with a different color) in aqueous solution with separate ligand binding sites in each subunit <ref name="InhA"> Rozwarski, D.A. ''et al.'' (1999). Crystal Structure of the ''Mycobacterium tuberculosis'' Enoyl-ACP Reductase, InhA, in Complex with NAD<sup>+</sup> and a C16 Fatty Acyl Substrate. ''Journal of Biological Chemistry, 274(22),'' 15582-15589. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/10336454 10336454] DOI: [http://www.ncbi.nlm.nih.gov/pubmed/10336454 10.1074/jbc.274.22.15582]</ref>. Each <scene name='69/694241/Monomer_subunit_no_ligands/1'>monomer</scene> subunit is composed of 289 residues and features a typical [http://en.wikipedia.org/wiki/Rossmann_fold Rossmann fold] containing a single NADH binding site. The <scene name='69/694241/Secondary_structure_black/1'>secondary structure</scene> of InhA is made up of several alpha helices (pink), beta sheets (gold), and beta turns (white) <ref name="Phe149"> Bell, A.F. ''et al.''(2007). Evidence from Raman Spectroscopy That InhA , the Mycobacterial Enoyl Reductase, Modulates the Conformation of the NADH Cofactor to Promote Catalysis. ''Journal of the American Chemical Society, 129,'' 6425-6431. DOI: [http://pubs.acs.org/doi/abs/10.1021/ja068219m 10.1021/ja068219m]</ref>. This enzyme also features a fatty acyl binding crevice that accommodates the long-chain fatty acyl substrate needed to synthesize mycolic acid precursors. The <scene name='69/694241/Helix6_helix7_updated/1'>alpha-6 and alpha-7 helices</scene> of the InhA form one side of the fatty acyl binding crevice, referred to as the <scene name='69/694241/Monomer_subunit_196_219/2'>substrate binding loop</scene> (residues 196-219). One side of this crevice is open and exposed to solvent, which allows the substrates to access the binding pocket of this enzyme. The size of the substrate binding loop is a primary determinant of the ability of InhA to select for fatty acyl chains longer than 16 carbons to successfully produce mycolic acid precursors <ref name="InhA" />. | + | Crystal structures of InhA reveal a <scene name='69/694241/Homotetramer_subunits_labeled/1'>homotetramer</scene> (each subunit featured with a different color) in aqueous solution with separate ligand binding sites in each subunit <ref name="InhA"> Rozwarski, D.A. ''et al.'' (1999). Crystal Structure of the ''Mycobacterium tuberculosis'' Enoyl-ACP Reductase, InhA, in Complex with NAD<sup>+</sup> and a C16 Fatty Acyl Substrate. ''Journal of Biological Chemistry, 274(22),'' 15582-15589. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/10336454 10336454] DOI: [http://www.ncbi.nlm.nih.gov/pubmed/10336454 10.1074/jbc.274.22.15582]</ref>. Each <scene name='69/694241/Monomer_subunit_no_ligands/1'>monomer</scene> subunit is composed of 289 residues and features a typical [http://en.wikipedia.org/wiki/Rossmann_fold Rossmann fold] (<scene name='69/694241/Rossmann_fold_better/1'>Rossmann Fold</scene> - beta sheets = light blue; alpha helices = dark blue) containing a single NADH binding site. The <scene name='69/694241/Secondary_structure_black/1'>secondary structure</scene> of InhA is made up of several alpha helices (pink), beta sheets (gold), and beta turns (white) <ref name="Phe149"> Bell, A.F. ''et al.''(2007). Evidence from Raman Spectroscopy That InhA , the Mycobacterial Enoyl Reductase, Modulates the Conformation of the NADH Cofactor to Promote Catalysis. ''Journal of the American Chemical Society, 129,'' 6425-6431. DOI: [http://pubs.acs.org/doi/abs/10.1021/ja068219m 10.1021/ja068219m]</ref>. This enzyme also features a fatty acyl binding crevice that accommodates the long-chain fatty acyl substrate needed to synthesize mycolic acid precursors. The <scene name='69/694241/Helix6_helix7_updated/1'>alpha-6 and alpha-7 helices</scene> of the InhA form one side of the fatty acyl binding crevice, referred to as the <scene name='69/694241/Monomer_subunit_196_219/2'>substrate binding loop</scene> (residues 196-219). One side of this crevice is open and exposed to solvent, which allows the substrates to access the binding pocket of this enzyme. The size of the substrate binding loop is a primary determinant of the ability of InhA to select for fatty acyl chains longer than 16 carbons to successfully produce mycolic acid precursors <ref name="InhA" />. |
==='''Ligands'''=== | ==='''Ligands'''=== | ||
Revision as of 13:45, 14 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Contents |
Enoyl-ACP Reductase InhA from Mycobacterium tuberculosis
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Bell, A.F. et al.(2007). Evidence from Raman Spectroscopy That InhA , the Mycobacterial Enoyl Reductase, Modulates the Conformation of the NADH Cofactor to Promote Catalysis. Journal of the American Chemical Society, 129, 6425-6431. DOI: 10.1021/ja068219m
- ↑ 2.0 2.1 Bhatt, A. et al. (2007). The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. Journal of Molecular Microbiology, 64(6), 1442-1454. PMID: 17555433 DOI: 10.1111/j.1365-2958.2007.05761.x
- ↑ Marrakchi, Hedia, et al. (2000). InhA, a target of the antituberculous drug isoniazid, is involved in a mycobacterial fatty acid elongation system, FAS-II. Journal of Microbiology, 146, 289-296. PMID: 10708367
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 Rozwarski, D.A. et al. (1999). Crystal Structure of the Mycobacterium tuberculosis Enoyl-ACP Reductase, InhA, in Complex with NAD+ and a C16 Fatty Acyl Substrate. Journal of Biological Chemistry, 274(22), 15582-15589. PMID: 10336454 DOI: 10.1074/jbc.274.22.15582
- ↑ 5.0 5.1 5.2 Vilchèze, C. et al. (2011). Novel Inhibitors of InhA Efficiently Kill Mycobacterium tuberculosis under Aerobic and Anaerobic Conditions. Antimicrobial Agents and Chemotherapy, 55(8), 3889-3898. DOI: 10.1128/AAC.00266-11
Student Contributors
- Arielle Russell
- Mackenzie A. Smith
Similar Proteopedia Pages
Enoyl-Acyl-Carrier Protein Reductase
Additional 3D Structures of Enoyl-ACP Reductase InhA
3oew, 2x22, 2x23, 1eny, 1enz, 4dqu, 4dre - MtENR+NAD; 3of2, 4dti - MtENR(mutant)+NAD; 2pr2, 2idz, 2h9i - MtENR+INH-NAPD; 2aq8 - MtENR+NADH; 2aqh, 2aqi, 2aqk, 3oey - MtENR(mutant)+NADH; 2ntj - MtENR+PTH-NAD; 2ie0, 2ieb, 2nv6, 1zid - MtENR(mutant)+INH-NAPD; 3fne, 3fnf, 3fng, 3fnh, 2b35, 1p45 - MtENR+NAD+TCI; 2b36, 2b37, 4ohu, 4oim, 4oyr - MtENR+NAD+phenoxyphenol derivative; 2nsd - MtENR+NAD+piperidine derivative; 2h7l, 2h7m, 2h7n, 2h7p, 4u0j, 4tzt, 4tzk, 4trj, 4u0k - MtENR+NAD+pyrrolidine derivative; 4cod, 4bqp, 4bqr, 4bge, 4bii, 4oxk, 4oxn, 4oxy, 4r9r, 4r9s - MtENR+NAD + inhibitor; 4bgi - MtENR (mutant)+NAD+inhibitor; 1p44 - MtENR+NAD+indole derivative; 1bvr - MtENR+NAD+fatty-acyl substrate