We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1058
From Proteopedia
(Difference between revisions)
| Line 52: | Line 52: | ||
==Mechanism of Action== | ==Mechanism of Action== | ||
[[Image:TCA_Cycle.png|500 px|left|thumb|'''Figure 5. Citric Acid Cycle with Glyoxylate Shunt Pathway.''' In several bacterial species, there is a carbon conserving gloxylate shunt pathway that converts isocitrate to malate in two steps instead of the usual five steps.]] | [[Image:TCA_Cycle.png|500 px|left|thumb|'''Figure 5. Citric Acid Cycle with Glyoxylate Shunt Pathway.''' In several bacterial species, there is a carbon conserving gloxylate shunt pathway that converts isocitrate to malate in two steps instead of the usual five steps.]] | ||
| + | |||
| + | |||
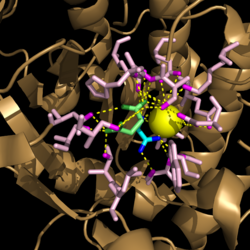
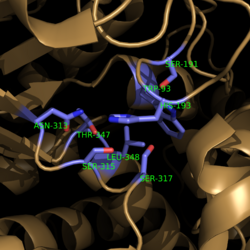
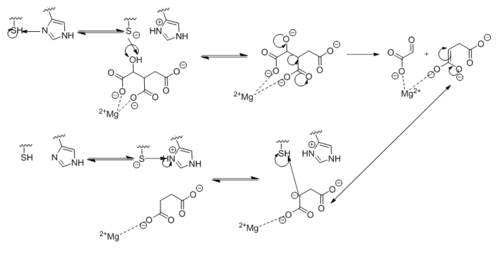
[[Image:Complete_Mechanism.PNG|500 px|right|thumb|'''Figure 6. Observed Mechanism for the Breakdown of Isocitrate by Isocitrate Lyase.''' His193 shifts the pKa of Cys191 and removes its proton. This allows Cys191 to extract a proton from the hydroxyl group of isocitrate. The resulting oxyanion forms a carbonyl and forces the lysis of a C-C bond. Glyoxylate and the enol form of succinate are formed and stabilized with a Mg<sup>2+</sup> ion. The succinate enolate resonates and extracts the proton back from Cys191 to form succinate.]] | [[Image:Complete_Mechanism.PNG|500 px|right|thumb|'''Figure 6. Observed Mechanism for the Breakdown of Isocitrate by Isocitrate Lyase.''' His193 shifts the pKa of Cys191 and removes its proton. This allows Cys191 to extract a proton from the hydroxyl group of isocitrate. The resulting oxyanion forms a carbonyl and forces the lysis of a C-C bond. Glyoxylate and the enol form of succinate are formed and stabilized with a Mg<sup>2+</sup> ion. The succinate enolate resonates and extracts the proton back from Cys191 to form succinate.]] | ||
| + | |||
| + | |||
==Disease Association== | ==Disease Association== | ||
| + | |||
| + | |||
| + | |||
===Clinical Implications=== | ===Clinical Implications=== | ||
''Mycobacterium tuberculosis'' is a respiratory infection that causes numerous fatalities throughout the world. It lives in organisms and feeds off of host cells, which indicate a variety of lipases exist within ''M. tuberculosis''. Current drugs that are on the market now target a small number of bacterial processes like cell wall formation and chromosomal replication. Although several antibiotics exist, all of them target these same mechanisms of inhibition. These commonalities have led to the prevalence of different multi-drug resistant (MDR) tuberculosis strains. Due to the high level of resistance, finding a lasting treatment for MDR TB infections has become very problematic. Studies into new mechanisms of inhibition will be crucial to prevent widespread outbreaks. | ''Mycobacterium tuberculosis'' is a respiratory infection that causes numerous fatalities throughout the world. It lives in organisms and feeds off of host cells, which indicate a variety of lipases exist within ''M. tuberculosis''. Current drugs that are on the market now target a small number of bacterial processes like cell wall formation and chromosomal replication. Although several antibiotics exist, all of them target these same mechanisms of inhibition. These commonalities have led to the prevalence of different multi-drug resistant (MDR) tuberculosis strains. Due to the high level of resistance, finding a lasting treatment for MDR TB infections has become very problematic. Studies into new mechanisms of inhibition will be crucial to prevent widespread outbreaks. | ||
Revision as of 02:57, 10 April 2015
Isocitrate Lyase from Mycobacterium tuberculosis
| |||||||||||
References
- ↑ Cozzone, A.; Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annual Review of Microbiology. 1998, 52:127-164. doi: 10.1146/annurev.micro.52.1.127.
- ↑ Srivastava, V.; Janin, A.; Srivastava, B.; Srivastava, R.; Selection of genes of Mycobacterium tuberculosis upregulated during residence in lungs of infected mice. ScienceDirect. 2007. doi:10.1016/j.tube.2007.10.002.
- ↑ Muñoz-Elías, E.; McKinney, J.; M. tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 2005. 11(6):638-644. doi:10.1038/nm1252.
- ↑ Dunn, M.; Ramírez-Trujillo, J.; Hernández-Lucas, I.; Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology. 2009. 155:3166-3175. doi:10.1099/mic.0.030858-0.