We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1070
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
===N-terminal Domain=== | ===N-terminal Domain=== | ||
| - | The N-terminal domain of MgtC is highly-conserved between [http://en.wiktionary.org/wiki/orthologue orthologues] of the MgtC super family. This domain is largely hydrophobic and serves as the main component of MgtC that allows its embedment in the extracellular membrane. While this domain is highly conserved amongst orthologues, a crystal structure is not yet available, but the sequence available has determined it to be largely hydrophobic. <ref name="mgtc"/> | + | The N-terminal domain of MgtC is highly-conserved between [http://en.wiktionary.org/wiki/orthologue orthologues] of the MgtC super family. This domain is largely hydrophobic and serves as the main component of MgtC that allows its embedment in the extracellular membrane. While this domain is highly conserved amongst orthologues, a [http://en.wikipedia.org/wiki/Crystal_structure crystal structure] is not yet available, but the sequence available has determined it to be largely hydrophobic. <ref name="mgtc"/> |
===C-terminal Domain=== | ===C-terminal Domain=== | ||
| - | This domain of MgtC, in contrast, is highly variable in comparison to several orthologues, as presented by Yang et al. However, through a sequence alignment of five known functional MgtC orthologues from [http://en.wikipedia.org/wiki/Pathogen pathogens] that survive inside macrophages (''M. tuberculosis, B. melitensis, B. cenocepacia, Y. pestis,'' and ''S. Typhimurium''), seven strictly conserved residues were found to be scattered along the whole sequence of the relatively hydrophilic and soluble C-terminal domain. <ref name="mgtc"/> | + | This domain of MgtC, in contrast, is highly variable in comparison to several orthologues, as presented by Yang et al. However, through a sequence alignment of five known functional MgtC orthologues from [http://en.wikipedia.org/wiki/Pathogen pathogens] that survive inside [http://en.wikipedia.org/wiki/Macrophage macrophages] (''M. tuberculosis, [http://en.wikipedia.org/wiki/Brucella_melitensis B. melitensis], [http://en.wikipedia.org/wiki/Burkholderia_cenocepacia B. cenocepacia], [http://en.wikipedia.org/wiki/Yersinia_pestis Y. pestis],'' and ''[http://en.wikipedia.org/wiki/Salmonella_enterica_subsp._enterica S. Typhimurium]''), seven strictly conserved residues were found to be scattered along the whole sequence of the relatively hydrophilic and soluble C-terminal domain. <ref name="mgtc"/> |
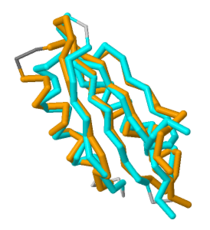
A large hydrophobic core has conserved residues <scene name='69/698113/Conserved_core_residues/6'>Cysteine-155, Arginine-164, Glutamine-160, and Alanine-195</scene>. | A large hydrophobic core has conserved residues <scene name='69/698113/Conserved_core_residues/6'>Cysteine-155, Arginine-164, Glutamine-160, and Alanine-195</scene>. | ||
| Line 59: | Line 59: | ||
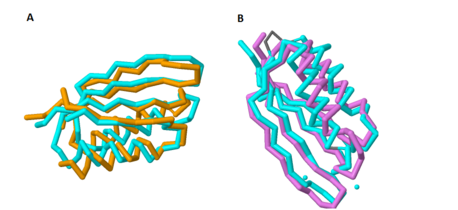
| - | Additionally, there is a crystal structure available for this domain. When comparing the crystal structure of the C-terminal domain to other protein structures, there are striking similarities between this domain and a class of proteins known as ACT domains. <ref name="mgtc"/> | + | Additionally, there is a crystal structure available for this domain. When comparing the crystal structure of the C-terminal domain to other protein structures, there are striking similarities between this domain and a class of proteins known as [http://en.wikipedia.org/wiki/ACT_domain ACT domains]. <ref name="mgtc"/> |
==Function== | ==Function== | ||
| Line 66: | Line 66: | ||
===Magnesium Transport=== | ===Magnesium Transport=== | ||
A role for MgtC as a magnesium transporter has been debated since its discovery. Several publications have produced data indicating that this protein is critical for the uptake of magnesium in magnesium-deprived medium, while other literature has shown that this protein plays an insignificant role in this process. | A role for MgtC as a magnesium transporter has been debated since its discovery. Several publications have produced data indicating that this protein is critical for the uptake of magnesium in magnesium-deprived medium, while other literature has shown that this protein plays an insignificant role in this process. | ||
| - | Support for a role in magnesium transport is supported by: 1) Mutants of MgtC are unable to survive in low-magnesium environment; 2) Expression of the gene encoding for MgtC is highly-induced in low magnesium environment; 3) Genes adjacent to the MgtC gene encode for known magnesium transporters. Very recent evidence against MgtC playing a role in magnesium transport showed that RT-PCR experiments gave consistent levels of MgtC expression despite changes in the concentration of extracellular magnesium. <ref name="mgtc"/> | + | Support for a role in magnesium transport is supported by: 1) Mutants of MgtC are unable to survive in low-magnesium environment; 2) Expression of the gene encoding for MgtC is highly-induced in low magnesium environment; 3) Genes adjacent to the MgtC gene encode for known magnesium transporters. Very recent evidence against MgtC playing a role in magnesium transport showed that [http://en.wikipedia.org/wiki/Reverse_transcription_polymerase_chain_reaction RT-PCR] experiments gave consistent levels of MgtC expression despite changes in the concentration of extracellular magnesium. <ref name="mgtc"/> |
Revision as of 23:41, 10 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
MgtC: A Virulence Factor From Mycobacterium tuberculosis
| |||||||||||
References
- ↑ Singh, G.; Singh, G.; Jadeja, D.; Kaur, J. Lipid hydrolyzing enzymes in virulence: Mycobacterium tuberculosis as a model system. Critical Reviews in Microbiology 2010, 36(3): 259-269.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 Yang, Y.; Labesse, G.; Carrere-Kremer, S.; Esteves, K.; Kremer, L.; Cohen-Gonsaud, M.; Blanc-Potard, A. The C-terminal domain of the virulence factor mgtc is a divergent act domain. J Bacteriol. 2012, 194(22): 6255-6263.
- ↑ 3.0 3.1 Jean-Francois, F.L.; Dai, J.; Yu, L. ; Myrick, A. ; Rubin, E. ; et al. Binding of mgtr, a salmonella transmembrane regulatory peptide, to mgtc, a mycobacterium tuberculosis virulence factor: a structural study.