Sandbox Reserved 1068
From Proteopedia
| Line 21: | Line 21: | ||
== Disease == | == Disease == | ||
| - | ''Mycobacterium tuberculosis'' is the causative agent of Tuberculosis (TB), an infectious disease that affects one-third of the worlds population. Two TB-related conditions exist: latent TB infection and active TB disease. Currently, there are four regimens that are approved for the treatment of latent TB infection through the use of the antibiotics isoniazid, rifampin, and rifapentine.TB disease can also be treated through various antibiotic regimens. There are 10 drugs currently approved by the FDA for treating TB disease. The first-line anti-TB agents are the antibiotics isoniazid, rifampin, ethambutol, and pyrazinamide.'''(CDC)''' Although various treatments for TB infection and TB disease exist, the emergence of multi-drug and extensively-drug resistant strains of ''M. tuberculosis'' has increased the need for anti-tubercular agents with novel modes of action. Iron is essential for mycobacterial growth and pathogenesis, ''M. tuberculosis'' obtains iron through mycobactin | + | ''Mycobacterium tuberculosis'' is the causative agent of Tuberculosis (TB), an infectious disease that affects one-third of the worlds population. Two TB-related conditions exist: latent TB infection and active TB disease. Currently, there are four regimens that are approved for the treatment of latent TB infection through the use of the antibiotics isoniazid, rifampin, and rifapentine.TB disease can also be treated through various antibiotic regimens. There are 10 drugs currently approved by the FDA for treating TB disease. The first-line anti-TB agents are the antibiotics isoniazid, rifampin, ethambutol, and pyrazinamide.'''(CDC)''' Although various treatments for TB infection and TB disease exist, the emergence of multi-drug and extensively-drug resistant strains of ''M. tuberculosis'' has increased the need for anti-tubercular agents with novel modes of action. |
| + | |||
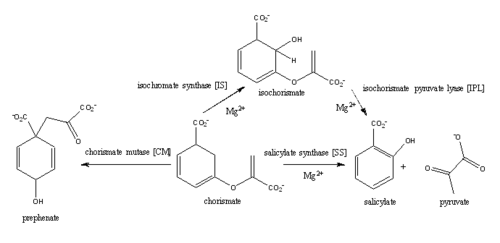
| + | Iron is essential for mycobacterial growth and pathogenesis, therefore the pathways for iron acquisition are potential targets for antibacterial therapies.''M. tuberculosis'' obtains iron through two different pathways: chelating iron from the host through the siderophore mycobactin and the degradation of heme released from damaged red blood cells''' (Reference).'''Mycobactin is a siderophore synthesized by the proteins encoded by the ''mbt'' and ''mbt2'' gene cluters (Reference). MbtI is the first enzyme in the mycobactin biosynthesis pathway and is a potential target for inhibition. The salicylate synthase activity of MbtI produces salicylate and pyruvate from chorismate through an isochorismate intermediate. Inhibition of MbtI activity would decrease the production of salicylate and therefore the synthesis of mycobactin; leading to a decrease in iron acquisition and pathogenesis of ''M. tuberculosis''. | ||
| + | |||
[[Image:Screen Shot 2015-04-10 at 1.27.15 PM.png|500 px|center|thumb|Figure 3: Reaction catalyzed by MbtI in the mycobactin biosynthesis pathway.]] | [[Image:Screen Shot 2015-04-10 at 1.27.15 PM.png|500 px|center|thumb|Figure 3: Reaction catalyzed by MbtI in the mycobactin biosynthesis pathway.]] | ||
| Line 47: | Line 50: | ||
Currently, isochorismate is believed to be formed from chorismate through a proposed Sn2 mechanism involving nucleophilic attack of an activated water molecule to the C2 center followed by either a concerted or stepwise elimination of the C4 hydroxyl group'''(He et al.)'''. Lys205 has been proposed to act as the catalytic base, activating a water molecule in the active site by abstracting one of its protons. However, mutational analysis of Lys205 suggested that the lysine reside is not the sole determinant in the activation of a water molecule for nucleophilic attack of the C2 center. Studies have shown that Lys205 is protonated at neutral pH and therefore can't act as a base to activate the water molecule, agreeing with the mutational analysis data. Instead of Lys205, Glu297 residue has been proposed to act as a base in the activation of the water molecule. The magnesium ion forces the negatively charged Glu297 residue to face toward the active site and the pKa of Glu297 (3.9) suggest an unprotonated state. Furthermore, Glu297 forms a hydrogen bond with a water molecule within the active site as well as with Lys205, which is in turn hydrogen bonded to C1 carboxylate group of chorismate and the oxygen of the nucleophilic water molecule. The glutamic residue (Gly252) could protonate the C4 leaving hydroxyl group. The pKa of Gly252 (7.7) suggest that is it is the only protonated glutamate residue in the active site at pH 7 and thus able to protonate the C4 leaving group. The pKa of Gly252 also accounts for the accumulation of isochorismate at pH values below 7.5. | Currently, isochorismate is believed to be formed from chorismate through a proposed Sn2 mechanism involving nucleophilic attack of an activated water molecule to the C2 center followed by either a concerted or stepwise elimination of the C4 hydroxyl group'''(He et al.)'''. Lys205 has been proposed to act as the catalytic base, activating a water molecule in the active site by abstracting one of its protons. However, mutational analysis of Lys205 suggested that the lysine reside is not the sole determinant in the activation of a water molecule for nucleophilic attack of the C2 center. Studies have shown that Lys205 is protonated at neutral pH and therefore can't act as a base to activate the water molecule, agreeing with the mutational analysis data. Instead of Lys205, Glu297 residue has been proposed to act as a base in the activation of the water molecule. The magnesium ion forces the negatively charged Glu297 residue to face toward the active site and the pKa of Glu297 (3.9) suggest an unprotonated state. Furthermore, Glu297 forms a hydrogen bond with a water molecule within the active site as well as with Lys205, which is in turn hydrogen bonded to C1 carboxylate group of chorismate and the oxygen of the nucleophilic water molecule. The glutamic residue (Gly252) could protonate the C4 leaving hydroxyl group. The pKa of Gly252 (7.7) suggest that is it is the only protonated glutamate residue in the active site at pH 7 and thus able to protonate the C4 leaving group. The pKa of Gly252 also accounts for the accumulation of isochorismate at pH values below 7.5. | ||
| - | '''isochorismate pyruvate lyase (IPL)''' | ||
'''salicylate synthase (SS)''' | '''salicylate synthase (SS)''' | ||
'''chorismate mutase (CM)''' | '''chorismate mutase (CM)''' | ||
Revision as of 02:15, 11 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Mycobacterium tuberculosis salicylate synthase (Mbt1)
| |||||||||||
References
1. Chi G1, Manos-Turvey A, O'Connor PD, Johnston JM, Evans GL, Baker EN, Payne RJ, Lott JS, Bulloch EM. 2012. Implications of binding mode and active site flexibility for inhibitor potency against the salicylate synthase from Mycobacterium tuberculosis. Biochemistry 51(24):4868-79. doi: 10.1021/bi3002067
2. Ferrer S1, Martí S, Moliner V, Tuñón I, Bertrán J. 2012 Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 14(10):3482-9. doi: 10.1039/c2cp23149b.
3. Harrison AJ1, Yu M, Gårdenborg T, Middleditch M, Ramsay RJ, Baker EN, Lott JS. 2006. The structure of MbtI from Mycobacterium tuberculosis, the first enzyme in the biosynthesis of the siderophore mycobactin, reveals it to be a salicylate synthase. J Bacteriol. 188(17):6081-91.
4. Manos-Turvey A1, Cergol KM, Salam NK, Bulloch EM, Chi G, Pang A, Britton WJ, West NP, Baker EN, Lott JS, Payne RJ. 2012. Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site. Org Biomol Chem 10(46):9223-36. doi: 10.1039/c2ob26736e.
5. Zwahlen J1, Kolappan S, Zhou R, Kisker C, Tonge PJ. 2007. Structure and mechanism of MbtI, the salicylate synthase from Mycobacterium tuberculosis. Biochemistry. 46(4):954-64.