We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1068
From Proteopedia
(Difference between revisions)
| Line 43: | Line 43: | ||
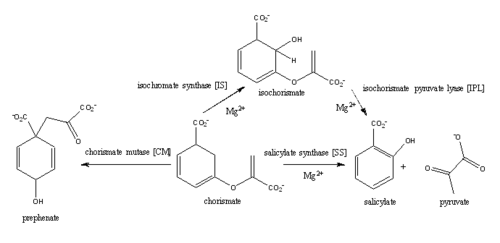
'''Isochorismate synthast (IS)''' | '''Isochorismate synthast (IS)''' | ||
| - | The C1 carboxylate group of chorismate binds to the magnesium cation within the active site. | ||
Currently, isochorismate is believed to be formed from chorismate through a proposed Sn2 mechanism involving nucleophilic attack of an activated water molecule to the C2 center followed by either a concerted or stepwise elimination of the C4 hydroxyl group <ref>PMID:14982443</ref>. Lys205 has been proposed to act as the catalytic base, activating a water molecule in the active site by abstracting one of its protons. However, mutational analysis of Lys205 suggested that the lysine reside is not the sole determinant in the activation of a water molecule for nucleophilic attack of the C2 center. Studies have shown that Lys205 is protonated at neutral pH and therefore can't act as a base to activate the water molecule, agreeing with the mutational analysis data. Instead of Lys205, Glu297 residue has been proposed to act as a base in the activation of the water molecule. The magnesium ion forces the negatively charged Glu297 residue to face toward the active site and the pKa of Glu297 (3.9) suggest an unprotonated state. Furthermore, Glu297 forms a hydrogen bond with a water molecule within the active site as well as with Lys205, which is in turn hydrogen bonded to C1 carboxylate group of chorismate and the oxygen of the nucleophilic water molecule. The glutamic residue (Gly252) could protonate the C4 leaving hydroxyl group. The pKa of Gly252 (7.7) suggest that is it is the only protonated glutamate residue in the active site at pH 7 and thus able to protonate the C4 leaving group. The pKa of Gly252 also accounts for the accumulation of isochorismate at pH values below 7.5. | Currently, isochorismate is believed to be formed from chorismate through a proposed Sn2 mechanism involving nucleophilic attack of an activated water molecule to the C2 center followed by either a concerted or stepwise elimination of the C4 hydroxyl group <ref>PMID:14982443</ref>. Lys205 has been proposed to act as the catalytic base, activating a water molecule in the active site by abstracting one of its protons. However, mutational analysis of Lys205 suggested that the lysine reside is not the sole determinant in the activation of a water molecule for nucleophilic attack of the C2 center. Studies have shown that Lys205 is protonated at neutral pH and therefore can't act as a base to activate the water molecule, agreeing with the mutational analysis data. Instead of Lys205, Glu297 residue has been proposed to act as a base in the activation of the water molecule. The magnesium ion forces the negatively charged Glu297 residue to face toward the active site and the pKa of Glu297 (3.9) suggest an unprotonated state. Furthermore, Glu297 forms a hydrogen bond with a water molecule within the active site as well as with Lys205, which is in turn hydrogen bonded to C1 carboxylate group of chorismate and the oxygen of the nucleophilic water molecule. The glutamic residue (Gly252) could protonate the C4 leaving hydroxyl group. The pKa of Gly252 (7.7) suggest that is it is the only protonated glutamate residue in the active site at pH 7 and thus able to protonate the C4 leaving group. The pKa of Gly252 also accounts for the accumulation of isochorismate at pH values below 7.5. | ||
Revision as of 07:15, 11 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Mycobacterium tuberculosis salicylate synthase (Mbt1)
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 3.2 Manos-Turvey A, Cergol KM, Salam NK, Bulloch EM, Chi G, Pang A, Britton WJ, West NP, Baker EN, Lott JS, Payne RJ. Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site. Org Biomol Chem. 2012 Dec 14;10(46):9223-36. doi: 10.1039/c2ob26736e. Epub 2012, Oct 29. PMID:23108268 doi:http://dx.doi.org/10.1039/c2ob26736e
- ↑ 4.0 4.1 4.2 4.3 Chi G, Manos-Turvey A, O'Connor PD, Johnston JM, Evans GL, Baker EN, Payne RJ, Lott JS, Bulloch EM. Implications of Binding Mode and Active Site Flexibility for Inhibitor Potency against the Salicylate Synthase from Mycobacterium tuberculosis. Biochemistry. 2012 Jun 7. PMID:22607697 doi:10.1021/bi3002067
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Tuberculosis (TB). Ed. Sam Posner. Centers for Disease Control and Prevention, n.d. Web. 9 Apr. 2015.
- ↑ De Voss, James J., Kerry Rutter, Benjamin G. Schroeder, Hua Su, and YaQi Zhu. The salicylate-derived mycobacterium siderophore of Mycobacterium tuberculosis are essential for growth in macrophages. "Proceedings of the National Science Academy" 97.3 (2000): 1252-57. Web. 5 Apr. 2015.
- ↑ He Z, Stigers Lavoie KD, Bartlett PA, Toney MD. Conservation of mechanism in three chorismate-utilizing enzymes. J Am Chem Soc. 2004 Mar 3;126(8):2378-85. PMID:14982443 doi:http://dx.doi.org/10.1021/ja0389927