We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1068
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
==Introduction== | ==Introduction== | ||
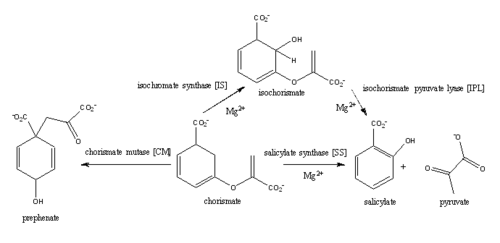
| - | Salicylate synthase from ''Mycobacterim tuberculosis'' (MtbI) is a highly promiscuous enzyme that has four distinct activities ''in vivo'': isochorismate synthase (IS), isochorismate pyruvate lyase (IPL), salicylate synthase (SS) and chromate mutate (CM). MtbI belongs to the chorismate-utilising enzyme family, which consists of structural homologues (<scene name='69/694235/Irp9/2'>Ipr9</scene>, <scene name='69/694235/Menf/2'>MenF</scene>, <scene name='69/694235/Entc/2'>EntC</scene>, and <scene name='69/694235/Mbti/2'>MbtI</scene>) that isomerize chromate to isochorismate. These enzymes are present in bacteria, fungi, plants and apicomplexan parasites and catalyze the initial reactions of menaquinone, siderophore, and tryptophan biosynthesis. The IS, IPL, and SS activity of MbtI require the presence of a magnesium ion within the active site, while CM activity is only observed in absence of the magnesium cation. IS, IPL, and SS activity are also modulated by the pH of the medium. Isochorismate is the primary product at pH values below 7.5 and salicylate is the primary product formed at pH 8. | + | Salicylate synthase from ''Mycobacterim tuberculosis'' (MtbI) is a highly promiscuous enzyme that has four distinct activities ''in vivo'': isochorismate synthase (IS), isochorismate pyruvate lyase (IPL), salicylate synthase (SS) and chromate mutate (CM). MtbI belongs to the chorismate-utilising enzyme family, which consists of structural homologues (<scene name='69/694235/Irp9/2'>Ipr9</scene>, <scene name='69/694235/Menf/2'>MenF</scene>, <scene name='69/694235/Entc/2'>EntC</scene>, and <scene name='69/694235/Mbti/2'>MbtI</scene>) that isomerize chromate to isochorismate. These enzymes are present in bacteria, fungi, plants and apicomplexan parasites and catalyze the initial reactions of menaquinone, siderophore, and tryptophan biosynthesis. The IS, IPL, and SS activity of MbtI require the presence of a magnesium ion within the active site, while CM activity is only observed in absence of the magnesium cation. IS, IPL, and SS activity are also modulated by the pH of the medium. Isochorismate is the primary product at pH values below 7.5 and salicylate is the primary product formed at pH 8. |
| - | + | The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating siderophore, mycobactin, in ''Mycobacterium tuberculosis'' (Figure 3)<ref name= "gamma chi">PMID:22607697</ref>. This complex secondary metabolite is essential for both virulence and survival of ''M. tuberculosis''. Therefore, inhibitors of salicylate synthase may serve as potential TB therapies with a novel mode of action(1,2,7,5,4,9) | |
| - | The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating siderophore, mycobactin, in ''Mycobacterium tuberculosis'' (Figure 3)<ref name= "gamma chi">PMID:22607697</ref>. This complex secondary metabolite is essential for both virulence and survival of ''M. tuberculosis''. Therefore, inhibitors of salicylate synthase may serve as potential TB therapies with a novel mode of action | + | |
| Line 15: | Line 14: | ||
==Structure== | ==Structure== | ||
[[Image:Active_site_cleft.png|300 px|left|thumb|Figure 2: This shows a single sub unit of MbtI, with the active site cleft located at the lower left hand side of the image.]] | [[Image:Active_site_cleft.png|300 px|left|thumb|Figure 2: This shows a single sub unit of MbtI, with the active site cleft located at the lower left hand side of the image.]] | ||
| - | The crystal asymmetric unit was found to contain <scene name='69/694235/3log/1'> four MbtI molecules</scene>, however crystal packing and size exclusion chromatography data suggest a monomeric enzyme. There are no significant structural changes between the four monomers excepts from the localized differences in the active site. The overall molecular structure consist of a polypeptide of 450 residues that forms one large single domain with a similar fold to other chromate-utilizing enzymes. The core of the protein is formed by 21 <scene name='69/694235/Beta_sheets/3'>beta-strands</scene> folded into a twisted beta-sandwich. The protein's core is then surrounded by 10 alpha helices. | + | The crystal asymmetric unit was found to contain <scene name='69/694235/3log/1'> four MbtI molecules</scene>, however crystal packing and size exclusion chromatography data suggest a monomeric enzyme. There are no significant structural changes between the four monomers excepts from the localized differences in the active site (3). The overall molecular structure consist of a polypeptide of 450 residues that forms one large single domain with a similar fold to other chromate-utilizing enzymes (3). The core of the protein is formed by 21 <scene name='69/694235/Beta_sheets/3'>beta-strands</scene> folded into a twisted beta-sandwich. The protein's core is then surrounded by 10 alpha helices(3). |
| Line 35: | Line 34: | ||
'''Magnesium cation effect''' | '''Magnesium cation effect''' | ||
| - | The presence of the magnesium ion induces changes in the structure of the active site and in the substrate, as well as causes significant pKa shifts in some of the key residues involved in the catalytic activity. The coordination shell of the magnesium cation in the active site of MbtI is composed of two water molecules, Glu434, Glu294, and the two oxygen atoms of the C1 carboxylate group of chorismate. In the presence of the magnesium ion, the positively charged Lys295 is displaced from the active site and the negatively charged Glu297 is faced toward the active site. Magnesium cation also orients the C1 carboxylate group coplanar to the ring of chorismate, reducing the electron density on the C2 center and favoring nucleophilic attack. | + | The presence of the magnesium ion induces changes in the structure of the active site and in the substrate, as well as causes significant pKa shifts in some of the key residues involved in the catalytic activity (8). The coordination shell of the magnesium cation in the active site of MbtI is composed of two water molecules, Glu434, Glu294, and the two oxygen atoms of the C1 carboxylate group of chorismate (8). In the presence of the magnesium ion, the positively charged Lys295 is displaced from the active site and the negatively charged Glu297 is faced toward the active site. Magnesium cation also orients the C1 carboxylate group coplanar to the ring of chorismate, reducing the electron density on the C2 center and favoring nucleophilic attack. |
'''Isochorismate pyruvae lyase (IPL)''' | '''Isochorismate pyruvae lyase (IPL)''' | ||
Revision as of 12:41, 14 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Mycobacterium tuberculosis salicylate synthase (Mbt1)
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 3.2 3.3 Chi G, Manos-Turvey A, O'Connor PD, Johnston JM, Evans GL, Baker EN, Payne RJ, Lott JS, Bulloch EM. Implications of Binding Mode and Active Site Flexibility for Inhibitor Potency against the Salicylate Synthase from Mycobacterium tuberculosis. Biochemistry. 2012 Jun 7. PMID:22607697 doi:10.1021/bi3002067
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Tuberculosis (TB). Ed. Sam Posner. Centers for Disease Control and Prevention, n.d. Web. 9 Apr. 2015.
- ↑ De Voss, James J., Kerry Rutter, Benjamin G. Schroeder, Hua Su, and YaQi Zhu. The salicylate-derived mycobacterium siderophore of Mycobacterium tuberculosis are essential for growth in macrophages. "Proceedings of the National Science Academy" 97.3 (2000): 1252-57. Web. 5 Apr. 2015.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedmanos-turvey - ↑ He Z, Stigers Lavoie KD, Bartlett PA, Toney MD. Conservation of mechanism in three chorismate-utilizing enzymes. J Am Chem Soc. 2004 Mar 3;126(8):2378-85. PMID:14982443 doi:http://dx.doi.org/10.1021/ja0389927