We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1063
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Introduction == | == Introduction == | ||
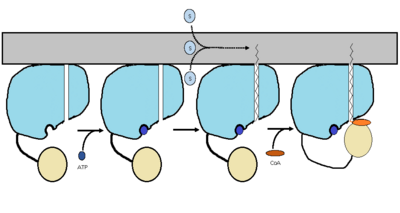
''<scene name='69/694230/Fadd13_subunits/3'>FadD13</scene>'' ''Mycobacterium Tuberculosis'' is an ACSVL (Acyl-CoA synthetases very long) peripheral membrane protein. ACS proteins activate [http://en.wikipedia.org/wiki/Lipid lipids] and [http://en.wikipedia.org/wiki/Fatty_acid fatty acids] before going into [http://en.wikipedia.org/wiki/Metabolic_pathway metabolic pathways]. FadD13 is soluble unlike other ACSVL proteins. FadD13 contains a hydrophobic tunnel for fatty acids to bind to, as well as an arginine rich lid loop that binds to the cell membrane. The binding of ATP causes structural changes promoting the binding of the hydrophobic substrates. Formation of an acyl-adenylate intermediate induces a 140 degree rotation of the small domain and binding of CoA for production of the final product, a fatty acyl-CoA thioester. Shown below is the general mechanism for ACS proteins. | ''<scene name='69/694230/Fadd13_subunits/3'>FadD13</scene>'' ''Mycobacterium Tuberculosis'' is an ACSVL (Acyl-CoA synthetases very long) peripheral membrane protein. ACS proteins activate [http://en.wikipedia.org/wiki/Lipid lipids] and [http://en.wikipedia.org/wiki/Fatty_acid fatty acids] before going into [http://en.wikipedia.org/wiki/Metabolic_pathway metabolic pathways]. FadD13 is soluble unlike other ACSVL proteins. FadD13 contains a hydrophobic tunnel for fatty acids to bind to, as well as an arginine rich lid loop that binds to the cell membrane. The binding of ATP causes structural changes promoting the binding of the hydrophobic substrates. Formation of an acyl-adenylate intermediate induces a 140 degree rotation of the small domain and binding of CoA for production of the final product, a fatty acyl-CoA thioester. Shown below is the general mechanism for ACS proteins. | ||
| - | [[Image:FadD13 steps.jpg| | + | [[Image:FadD13 steps.jpg|400 px|thumb|left|General Mechanism of ACSVL Enzymes]] |
== Background == | == Background == | ||
| Line 15: | Line 15: | ||
The FadD13 enzyme functions to activate lipids before going into metabolic pathways. This is done by ATP/AMP binding to the <scene name='69/694230/Atp_and_amp_binding_region/4'>ATP and AMP binding region</scene>. Once ATP/AMP is bound, the long lipid chain up to 26 carbons may bind in the hydrophobic portion of the enzyme. Upon binding of the substrate, the C terminal swings up to close off the tunnel. From there CoA can bind to produce the final product, an acyl-CoA Thioester. The lipid can now move transversely throughout the membrane and throughout the rest of the cell. Below is the proposed mechanism for ACSVL proteins<ref> Andersson, C.S., Lundgren, C.A.K., Magnusdottir, A., Ge, C., Weislander, A., Molina, D., Hogbom, M. (2012)The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: structural Basis for Housing lipid Substrates longer than the Enzyme. Cell Press,1062-1070 </ref>. | The FadD13 enzyme functions to activate lipids before going into metabolic pathways. This is done by ATP/AMP binding to the <scene name='69/694230/Atp_and_amp_binding_region/4'>ATP and AMP binding region</scene>. Once ATP/AMP is bound, the long lipid chain up to 26 carbons may bind in the hydrophobic portion of the enzyme. Upon binding of the substrate, the C terminal swings up to close off the tunnel. From there CoA can bind to produce the final product, an acyl-CoA Thioester. The lipid can now move transversely throughout the membrane and throughout the rest of the cell. Below is the proposed mechanism for ACSVL proteins<ref> Andersson, C.S., Lundgren, C.A.K., Magnusdottir, A., Ge, C., Weislander, A., Molina, D., Hogbom, M. (2012)The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: structural Basis for Housing lipid Substrates longer than the Enzyme. Cell Press,1062-1070 </ref>. | ||
| - | [[Image:Proposed Mechanism.png| | + | [[Image:Proposed Mechanism.png|400 px|thumb|left|Proposed Mechanism]] |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
{{reflist}} | {{reflist}} | ||
Revision as of 12:27, 14 April 2015
FadD13
| |||||||||||