We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1070
From Proteopedia
(Difference between revisions)
| Line 73: | Line 73: | ||
===Potential for Binding Amino Acids=== | ===Potential for Binding Amino Acids=== | ||
The exploration of this role for MgtC was first considered because of the ACT domain-like structure of the C-terminal domain. | The exploration of this role for MgtC was first considered because of the ACT domain-like structure of the C-terminal domain. | ||
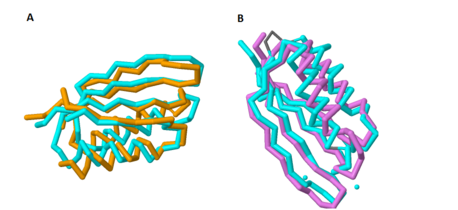
| - | ACT domains commonly bind small amino acids within the cell as a form of regulation. Yang ''et al''. showed that the structure | + | ACT domains commonly bind small amino acids within the cell as a form of [http://geneontology.org/page/regulation regulation]. Yang ''et al''. showed that the structure of the C-terminal domain overlaps significantly with the structure of [http://proteopedia.org/wiki/index.php/1psd SerA] (PDB: [http://www.rcsb.org/pdb/explore/explore.do?structureId=1psd 1PSD]), a known amino acid-binding ACT domain from ''[http://www.cdc.gov/ecoli/ E. coli]''. '''Figure 1A''' shows the overlap of these two proteins; the cyan protein represents MgtC and the orange protein represents SerA. However, the glycine that is critical for the binding of amino acids in these ACT domains has been substituted in MgtC with a <scene name='69/698113/Sub_residues_of_sera/1'>tyrosine</scene>, likely abolishing any potential amino acid binding activity <ref name="mgtc"/> |
| - | of the C-terminal domain overlaps significantly with the structure of [http://proteopedia.org/wiki/index.php/1psd SerA] (PDB: [http://www.rcsb.org/pdb/explore/explore.do?structureId=1psd 1PSD]), a known amino acid-binding ACT domain from ''[http://www.cdc.gov/ecoli/ E. coli]'' | + | |
| - | '''Figure 1A''' shows the overlap of these two proteins; the cyan protein represents MgtC and the orange protein represents SerA. | + | |
| - | However, the glycine that is critical for the binding of amino acids in these ACT domains has been substituted in MgtC with a <scene name='69/698113/Sub_residues_of_sera/1'>tyrosine</scene>, likely abolishing any potential amino acid binding activity <ref name="mgtc"/> | + | |
===Potential for Chelation=== | ===Potential for Chelation=== | ||
Revision as of 13:40, 14 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
MgtC: A Virulence Factor From Mycobacterium tuberculosis
| |||||||||||
References
- ↑ Singh, G.; Singh, G.; Jadeja, D.; Kaur, J. Lipid hydrolyzing enzymes in virulence: Mycobacterium tuberculosis as a model system. Critical Reviews in Microbiology 2010, 36(3): 259-269. DOI: 10.3109/1040841X.2010.482923.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Yang, Y.; Labesse, G.; Carrere-Kremer, S.; Esteves, K.; Kremer, L.; Cohen-Gonsaud, M.; Blanc-Potard, A. The C-terminal domain of the virulence factor mgtc is a divergent act domain. J Bacteriol. 2012, 194(22): 6255-6263. DOI: 10.1128/JB.01424-12.
- ↑ Blanc-Potard, A.B.; Lafay, B. MgtC as a horizontally-acquired virulence factor of intracellular bacterial pathogens : evidence from molecular phylogeny and comparative genomics. J Mol Evol. 2003, 57(4): 479-86. DOI: 10.1007/s00239-003-2496-4
- ↑ Belon, C.; Gannoun-Zaki, L.; Lutfalla, G.; Kremer, L.; Blanc-Potard, A.B. Mycobacterium marinum mgtc plays a role in phagocytosis but is dispensable for intracellular multiplication. Plos One 2014, 1-23. DOI: 10.1371/journal.pone.0116052.
- ↑ 5.0 5.1 5.2 Jean-Francois, F.L.; Dai, J.; Yu, L. ; Myrick, A. ; Rubin, E. ; et al. Binding of mgtr, a salmonella transmembrane regulatory peptide, to mgtc, a mycobacterium tuberculosis virulence factor: a structural study. DOI:10.1016/j.jmb.2013.10.014