We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1070
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='2lqj' size='340' side='right' caption='C-terminal Domain of Mg2+ transport P-type ATPase C (PDB: [http://www.rcsb.org/pdb/explore.do?structureId=2lqj 2LQJ])' scene='69/698113/Rainbow-colored_spectrum/2'> | <StructureSection load='2lqj' size='340' side='right' caption='C-terminal Domain of Mg2+ transport P-type ATPase C (PDB: [http://www.rcsb.org/pdb/explore.do?structureId=2lqj 2LQJ])' scene='69/698113/Rainbow-colored_spectrum/2'> | ||
==Introduction== | ==Introduction== | ||
| - | [http://en.wikipedia.org/wiki/Tuberculosis Tuberculosis], caused by ''[http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis]'', is a [http://en.wikipedia.org/wiki/Respiratory_tract_infection respiratory infection] still prevalent throughout the world. During the last decade, the emergence of [http://en.wikipedia.org/wiki/Multiple_drug_resistance multi-drug resistant] strains of ''M. tuberculosis'' has given rise to the need for the development of new antibiotics in order to combat the infection<ref>Singh, G.; Singh, G.; Jadeja, D.; Kaur, J. Lipid hydrolyzing enzymes in virulence: Mycobacterium tuberculosis as a model system. Critical Reviews in Microbiology 2010, 36(3): 259-269. DOI: 10.3109/1040841X.2010.482923.</ref>. In order to develop an efficacious antibiotic, the drug must be able to target a unique aspect of the bacteria, such as a protein, that is critical for its full virulence and survival. MgtC, an [http://en.wikipedia.org/wiki/Integral_membrane_protein integral protein] embedded in the extracellular membrane of ''M. tuberculosis'', has recently been hypothesized as a novel drug target to resolve tuberculosis infections. The targeting of MgtC was a result of observing that upon deletion of the protein from ''M. tuberculosis'', the bacteria are no longer able to survive due to inhibition of [http://en.wiktionary.org/wiki/intramacrophage intramacrophage] growth. <ref name="mgtc">Yang, Y.; Labesse, G.; Carrere-Kremer, S.; Esteves, K.; Kremer, L.; Cohen-Gonsaud, M.; Blanc-Potard, A. The C-terminal domain of the virulence factor mgtc is a divergent act domain. J Bacteriol. 2012, 194(22): 6255-6263. DOI: 10.1128/JB.01424-12.</ref>. | + | [http://en.wikipedia.org/wiki/Tuberculosis Tuberculosis], caused by ''[http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis]'', is a [http://en.wikipedia.org/wiki/Respiratory_tract_infection respiratory infection] still prevalent throughout the world. During the last decade, the emergence of [http://en.wikipedia.org/wiki/Multiple_drug_resistance multi-drug resistant] strains of ''M. tuberculosis'' has given rise to the need for the development of new [http://en.wikipedia.org/wiki/Antibiotics antibiotics] in order to combat the infection<ref>Singh, G.; Singh, G.; Jadeja, D.; Kaur, J. Lipid hydrolyzing enzymes in virulence: Mycobacterium tuberculosis as a model system. Critical Reviews in Microbiology 2010, 36(3): 259-269. DOI: 10.3109/1040841X.2010.482923.</ref>. In order to develop an efficacious antibiotic, the drug must be able to target a unique aspect of the bacteria, such as a protein, that is critical for its full virulence and survival. MgtC, an [http://en.wikipedia.org/wiki/Integral_membrane_protein integral protein] embedded in the extracellular membrane of ''M. tuberculosis'', has recently been hypothesized as a novel drug target to resolve tuberculosis infections. The targeting of MgtC was a result of observing that upon deletion of the protein from ''M. tuberculosis'', the bacteria are no longer able to survive due to inhibition of [http://en.wiktionary.org/wiki/intramacrophage intramacrophage] growth. <ref name="mgtc">Yang, Y.; Labesse, G.; Carrere-Kremer, S.; Esteves, K.; Kremer, L.; Cohen-Gonsaud, M.; Blanc-Potard, A. The C-terminal domain of the virulence factor mgtc is a divergent act domain. J Bacteriol. 2012, 194(22): 6255-6263. DOI: 10.1128/JB.01424-12.</ref>. |
== Structure == | == Structure == | ||
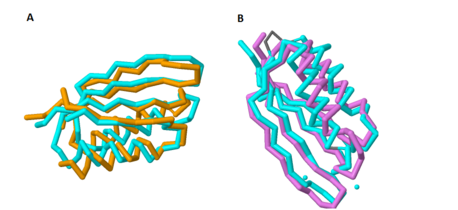
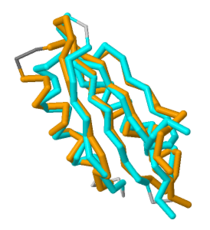
Based on its [http://en.wikipedia.org/wiki/Protein_tertiary_structure tertiary structure], this protein has been placed into a larger group of proteins known as the [http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi?uid=120498 MgtC superfamily]. The overall structure of MgtC is constituted by two [http://en.wikipedia.org/wiki/Protein_domain domains]: an N-terminal domain and a C-terminal domain. Each of these domains have striking similarities and differences with other MgtC-like proteins.<ref name="mgtc"/> | Based on its [http://en.wikipedia.org/wiki/Protein_tertiary_structure tertiary structure], this protein has been placed into a larger group of proteins known as the [http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi?uid=120498 MgtC superfamily]. The overall structure of MgtC is constituted by two [http://en.wikipedia.org/wiki/Protein_domain domains]: an N-terminal domain and a C-terminal domain. Each of these domains have striking similarities and differences with other MgtC-like proteins.<ref name="mgtc"/> | ||
| Line 83: | Line 83: | ||
==Clinical Relevance == | ==Clinical Relevance == | ||
| - | The development of an antibiotic which targets and inhibits MgtC could come from exploitation and enhancement of the process which promotes its degradation within ''Mycobacterium tuberculosis.'' MgtR, a hydrophobic peptide, promotes the degradation of MgtC upon high expression within the bacteria.<ref name="mgtr"/> As previously stated, inadequate levels of MgtC within ''M. tuberculosis'' results in an inability to | + | The development of an antibiotic which targets and inhibits MgtC could come from exploitation and enhancement of the process which promotes its degradation within ''Mycobacterium tuberculosis.'' MgtR, a hydrophobic peptide, promotes the degradation of MgtC upon high expression within the bacteria.<ref name="mgtr"/> As previously stated, inadequate levels of MgtC within ''M. tuberculosis'' results in an inability to grow and survive. <ref name="mgtr"/> It is quite reasonable that analogues of MgtR could be developed, injected ([http://en.wikipedia.org/wiki/Subcutaneous_injection subcutaneously]) into infected patients, and resolve the tuberculosis infection by promoting degradation of MgtC and impairing growth of ''M. tuberculosis.'' |
==Future Work== | ==Future Work== | ||
Revision as of 13:47, 14 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
MgtC: A Virulence Factor From Mycobacterium tuberculosis
| |||||||||||
References
- ↑ Singh, G.; Singh, G.; Jadeja, D.; Kaur, J. Lipid hydrolyzing enzymes in virulence: Mycobacterium tuberculosis as a model system. Critical Reviews in Microbiology 2010, 36(3): 259-269. DOI: 10.3109/1040841X.2010.482923.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Yang, Y.; Labesse, G.; Carrere-Kremer, S.; Esteves, K.; Kremer, L.; Cohen-Gonsaud, M.; Blanc-Potard, A. The C-terminal domain of the virulence factor mgtc is a divergent act domain. J Bacteriol. 2012, 194(22): 6255-6263. DOI: 10.1128/JB.01424-12.
- ↑ Blanc-Potard, A.B.; Lafay, B. MgtC as a horizontally-acquired virulence factor of intracellular bacterial pathogens : evidence from molecular phylogeny and comparative genomics. J Mol Evol. 2003, 57(4): 479-86. DOI: 10.1007/s00239-003-2496-4
- ↑ Belon, C.; Gannoun-Zaki, L.; Lutfalla, G.; Kremer, L.; Blanc-Potard, A.B. Mycobacterium marinum mgtc plays a role in phagocytosis but is dispensable for intracellular multiplication. Plos One 2014, 1-23. DOI: 10.1371/journal.pone.0116052.
- ↑ 5.0 5.1 5.2 Jean-Francois, F.L.; Dai, J.; Yu, L. ; Myrick, A. ; Rubin, E. ; et al. Binding of mgtr, a salmonella transmembrane regulatory peptide, to mgtc, a mycobacterium tuberculosis virulence factor: a structural study. DOI:10.1016/j.jmb.2013.10.014