This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1074
From Proteopedia
| Line 44: | Line 44: | ||
== '''Catalytic Triad''' == | == '''Catalytic Triad''' == | ||
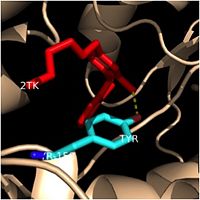
| - | Structural studies have shown that InhA possesses a <scene name='69/694241/ | + | Structural studies have shown that InhA possesses a <scene name='69/694241/Catalytic_triad_best/1'>Phe-Tyr-Lys catalytic triad</scene>, composed of Phe-149, Tyr-158, and Lys-165. This catalytic triad of InhA is analogous to the classic Ser-Tyr-Lys catalytic triad present in all members of the SDR (Short-chain Dehydrogenase Reductase) family <ref name="InhA" />. In the dehydrogenases, the first catalytic residue is usually Ser or Thr, while in the enoyl reuctases (InhA), the first catalytic residue is usually Phe or Tyr <ref name="Phe149" />. According to crystallographic data, the likely role of Tyr-158 is to stabilize the enolate intermediate that forms during the hydride transfer reaction <ref name="InhA" />. Previous structural and kinetic studies have confirmed that the side chain of the Lys-165 residue in InhA functions similarly to the catalytic Lys residues in other SDR enzymes. The catalytic Lys-165 residue in InhA interacts with the 2' or 3'-hydroxyls of the nicotinamide ring of NADH to hold this cofactor in place within the fatty acyl binding crevice <ref name="InhA" />. Finally, the exact role of the catalytic Phe-149 residue in InhA is less well known. Recent Raman spectroscopy studies have supported that the catalytic Phe-149 residue plays an essential role in orienting the NADH cofactor correctly to lower the energy of the transition state and promoting the hydride transfer reaction <ref name="Phe149" />. Altogether, the residues in the <scene name='69/694241/Catalytic_triad_in_structure/1'>catalytic triad</scene> of InhA play critical roles in properly orienting NADH and the fatty acyl substrate to promote the hydride transfer reaction required to elongate the fatty acyl chains and produce mycolic acid precursors. |
Revision as of 18:31, 14 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Contents |
Enoyl-ACP Reductase InhA from Mycobacterium tuberculosis
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Bell, A.F. et al.(2007). Evidence from Raman Spectroscopy That InhA , the Mycobacterial Enoyl Reductase, Modulates the Conformation of the NADH Cofactor to Promote Catalysis. Journal of the American Chemical Society, 129, 6425-6431. DOI: 10.1021/ja068219m
- ↑ 2.0 2.1 Bhatt, A. et al. (2007). The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. Journal of Molecular Microbiology, 64(6), 1442-1454. PMID: 17555433 DOI: 10.1111/j.1365-2958.2007.05761.x
- ↑ Marrakchi, Hedia, et al. (2000). InhA, a target of the antituberculous drug isoniazid, is involved in a mycobacterial fatty acid elongation system, FAS-II. Journal of Microbiology, 146, 289-296. PMID: 10708367
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 Rozwarski, D.A. et al. (1999). Crystal Structure of the Mycobacterium tuberculosis Enoyl-ACP Reductase, InhA, in Complex with NAD+ and a C16 Fatty Acyl Substrate. Journal of Biological Chemistry, 274(22), 15582-15589. PMID: 10336454 DOI: 10.1074/jbc.274.22.15582
- ↑ 5.0 5.1 5.2 Vilchèze, C. et al. (2011). Novel Inhibitors of InhA Efficiently Kill Mycobacterium tuberculosis under Aerobic and Anaerobic Conditions. Antimicrobial Agents and Chemotherapy, 55(8), 3889-3898. DOI: 10.1128/AAC.00266-11
Student Contributors
- Arielle Russell
- Mackenzie A. Smith
Similar Proteopedia Pages
Enoyl-Acyl-Carrier Protein Reductase
Additional 3D Structures of Enoyl-ACP Reductase InhA
3oew, 2x22, 2x23, 1eny, 1enz, 4dqu, 4dre - MtENR+NAD; 3of2, 4dti - MtENR(mutant)+NAD; 2pr2, 2idz, 2h9i - MtENR+INH-NAPD; 2aq8 - MtENR+NADH; 2aqh, 2aqi, 2aqk, 3oey - MtENR(mutant)+NADH; 2ntj - MtENR+PTH-NAD; 2ie0, 2ieb, 2nv6, 1zid - MtENR(mutant)+INH-NAPD; 3fne, 3fnf, 3fng, 3fnh, 2b35, 1p45 - MtENR+NAD+TCI; 2b36, 2b37, 4ohu, 4oim, 4oyr - MtENR+NAD+phenoxyphenol derivative; 2nsd - MtENR+NAD+piperidine derivative; 2h7l, 2h7m, 2h7n, 2h7p, 4u0j, 4tzt, 4tzk, 4trj, 4u0k - MtENR+NAD+pyrrolidine derivative; 4cod, 4bqp, 4bqr, 4bge, 4bii, 4oxk, 4oxn, 4oxy, 4r9r, 4r9s - MtENR+NAD + inhibitor; 4bgi - MtENR (mutant)+NAD+inhibitor; 1p44 - MtENR+NAD+indole derivative; 1bvr - MtENR+NAD+fatty-acyl substrate