We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1053
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
== Background == | == Background == | ||
| - | The antigen 85 (ag85) complex in Mycobacterium tuberculosis is composed of three intracellular membrane proteins: | + | The antigen 85 (ag85) complex in ''Mycobacterium tuberculosis'' is composed of three intracellular membrane proteins: Ag85A, B, and C. The Ag85 complex is a major component of the cell wall, with each protein catalyzing the transfer of important cell wall constituents into the membrane. <ref>PMID: 10655617</ref> Ag85C is of particular interest due to its transfer of mycolic acids, which are major components in determining cell wall integrity. By targeting this mycoloyltransferase activity, inhibition of Ag85C offers potential for cell wall disruption and subsequent antibiotic targeting for normally drug-resistant ''Mycotaberia tuberculosis''. <ref>PMID: 10200974</ref> |
==Structure== | ==Structure== | ||
| - | <StructureSection load='1dqz' size='400' side='right' caption='Antigen 85C in Mycobacterium Tuberculosis' scene=''> | + | <StructureSection load='1dqz' size='400' side='right' caption='Antigen 85C in ''Mycobacterium Tuberculosis''' scene=''> |
== General Structure == | == General Structure == | ||
| - | Antigen 85C was crystallized in its dimeric form.<ref>PMID: 25028518</ref> The <scene name='69/694220/Secondary_structures/2'>secondary structure</scene> shown in the monomeric form is composed of helices | + | Antigen 85C was crystallized in its dimeric form.<ref>PMID: 25028518</ref> The <scene name='69/694220/Secondary_structures/2'>secondary structure</scene> shown in the monomeric form is composed of helices, shown in pink, with one interwoven beta sheet, shown in yellow. The confrontation of a central β-sheet bordered by α–helices creates an a α/β hydrolase fold in Ag85C, and this tertiary conformation is highly conserved across enzymes that function in this capacity. <ref>PMID:10655617</ref> The substrate binding pocket of Ag85C is composed of two separate but equally important components; there is carbohydrate binding pocket for the trehalose, and there is a fatty acid binding pocket for the mycolic acid. As a result, trahalose monomycolate can effectively bind to the Ag85C binding pocket. |
== Enzymatic Activity == | == Enzymatic Activity == | ||
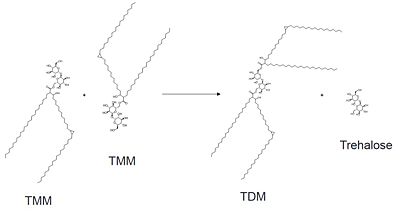
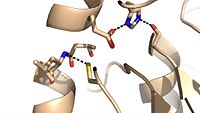
| - | Mutagenesis studies have confirmed the Ag85C functions through a Glu-His-Ser <scene name='69/694220/Catalytic_triad/4'>catalytic triad</scene>, similar to that of chymotrypsin. By modifying each of the catalytic residues separately testing the enzyme’s relative activity, it has been shown that mutation of any one of these residues dramatically reduces activity (Figure #). The S124 alcohol’s nucleophilicity is inductively strengthened through H260 and E224, which allows S124 to | + | Mutagenesis studies have confirmed the Ag85C functions through a Glu-His-Ser <scene name='69/694220/Catalytic_triad/4'>catalytic triad</scene>, similar to that of chymotrypsin. By modifying each of the catalytic residues separately testing the enzyme’s relative activity, it has been shown that mutation of any one of these residues dramatically reduces activity (Figure #). The S124 alcohol’s nucleophilicity is inductively strengthened through H260 and E224, which allows the S124 residue to catalyze a reaction that involves trehalose 6, 6’-dimycolate. The formation of the functional catalytic triad relies on upon Van der Waals interaction between C209 and the peptide bond between L232 and T231. This interaction results in a kinked conformation of the α9 helix, which promotes that activity of the catalytic triad. As a result, Ag85C, a mycolyl transferase, can facilitate the modification of trehalose monomycolates to trehalose dimycolates, which are then transported to the bacterial cell wall. |
[[Image:General_mechanism.jpg|400 px|center|General reaction catalyzed by Antigen 85C]] | [[Image:General_mechanism.jpg|400 px|center|General reaction catalyzed by Antigen 85C]] | ||
| Line 20: | Line 20: | ||
[[Image:C209.jpeg|200 px|left|thumb|Cys209 stabilizing kinked formation of alpha-9 helix]] | [[Image:C209.jpeg|200 px|left|thumb|Cys209 stabilizing kinked formation of alpha-9 helix]] | ||
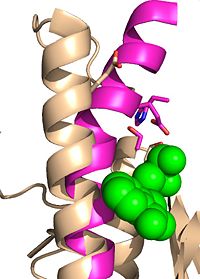
| - | Due to the importance of Ag85C enzymatic activity in maintaining the integrity of the | + | Due to the importance of Ag85C enzymatic activity in maintaining the integrity of the ''Mycobacteria tuberculosis'' cell wall though mycolic acid modifications, the Ag85C enzyme represents a potentially effective avenue for inhibiting cell growth. The conformational sensitivity of the active site residues, H260, E228, and S124, relies entirely upon Van der Waals interaction between C209 and L232-T 231 (Figure #). The C209 facilitated interaction causes the <scene name='69/694220/Alpha_9_helix/2'>α9 helix</scene> to acquire a kinked conformation that promotes optimal interaction distances between catalytic residues. As a result, C209 has been a specific target residue for Ag85C inhibition.> |
Revision as of 17:44, 17 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Background
The antigen 85 (ag85) complex in Mycobacterium tuberculosis is composed of three intracellular membrane proteins: Ag85A, B, and C. The Ag85 complex is a major component of the cell wall, with each protein catalyzing the transfer of important cell wall constituents into the membrane. [1] Ag85C is of particular interest due to its transfer of mycolic acids, which are major components in determining cell wall integrity. By targeting this mycoloyltransferase activity, inhibition of Ag85C offers potential for cell wall disruption and subsequent antibiotic targeting for normally drug-resistant Mycotaberia tuberculosis. [2]
Structure
| |||||||||||
References
- ↑ Ronning DR, Klabunde T, Besra GS, Vissa VD, Belisle JT, Sacchettini JC. Crystal structure of the secreted form of antigen 85C reveals potential targets for mycobacterial drugs and vaccines. Nat Struct Biol. 2000 Feb;7(2):141-6. PMID:10655617 doi:10.1038/72413
- ↑ Jackson M, Raynaud C, Laneelle MA, Guilhot C, Laurent-Winter C, Ensergueix D, Gicquel B, Daffe M. Inactivation of the antigen 85C gene profoundly affects the mycolate content and alters the permeability of the Mycobacterium tuberculosis cell envelope. Mol Microbiol. 1999 Mar;31(5):1573-87. PMID:10200974
- ↑ Favrot L, Lajiness DH, Ronning DR. Inactivation of the Mycobacterium tuberculosis Antigen 85 complex by covalent, allosteric inhibitors. J Biol Chem. 2014 Jul 14. pii: jbc.M114.582445. PMID:25028518 doi:http://dx.doi.org/10.1074/jbc.M114.582445
- ↑ Ronning DR, Klabunde T, Besra GS, Vissa VD, Belisle JT, Sacchettini JC. Crystal structure of the secreted form of antigen 85C reveals potential targets for mycobacterial drugs and vaccines. Nat Struct Biol. 2000 Feb;7(2):141-6. PMID:10655617 doi:10.1038/72413