We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1056
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
==Isocitrate Lyase from ''Mycobacterium Tuberculosis''== | ==Isocitrate Lyase from ''Mycobacterium Tuberculosis''== | ||
<StructureSection load='1F8I' size='340' side='right' caption='Isocitrate Lyase from ''Mycobacterium tuberculosis''' scene=''> | <StructureSection load='1F8I' size='340' side='right' caption='Isocitrate Lyase from ''Mycobacterium tuberculosis''' scene=''> | ||
| + | |||
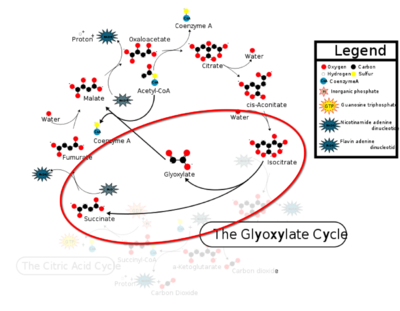
[[Image:CAC.png|400 px|right|thumb|Figure 1: ICL mediated glyoxylate shunt pathway of the Citric Acid Cycle]] | [[Image:CAC.png|400 px|right|thumb|Figure 1: ICL mediated glyoxylate shunt pathway of the Citric Acid Cycle]] | ||
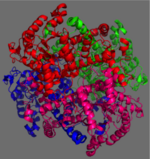
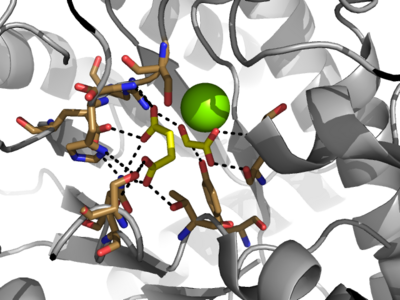
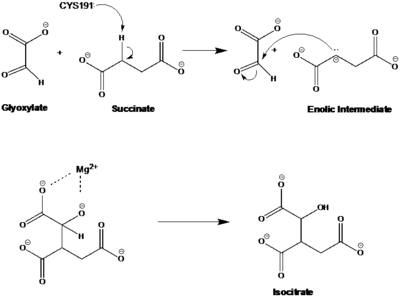
[http://en.wikipedia.org/wiki/Isocitrate_lyase Isocitrate Lyase] (ICL) is a metabolic enzyme that converts the metabolite isocitrate into glyoxylate and succinate. ICL is a homotetramer with each monomer being composed of 14 alpha helices, 14 beta sheets, and a magnesium ion cofactor. ICL has shown clinical relevance in the disease state [http://en.wikipedia.org/wiki/Tuberculosis Tuberculosis] where it is responsible for the persistence of ''Mycobacterium tuberculosis'' during the chronic stage of infection<ref name="genes">PMID: 18054522</ref> This survival strategy mediated by ICL is characterized by a metabolic shortcut within the [http://en.wikipedia.org/wiki/Citric_acid_cycle Citric Acid Cycle]. ICL creates this shunt pathway by converting isocitrate to succinate and glyoxylate, diverting acetyl-CoA from the beta-oxidation of fatty acids<ref name="ICL">PMID:10932251</ref><ref name="ICL2">PMID: 2696959</ref>. | [http://en.wikipedia.org/wiki/Isocitrate_lyase Isocitrate Lyase] (ICL) is a metabolic enzyme that converts the metabolite isocitrate into glyoxylate and succinate. ICL is a homotetramer with each monomer being composed of 14 alpha helices, 14 beta sheets, and a magnesium ion cofactor. ICL has shown clinical relevance in the disease state [http://en.wikipedia.org/wiki/Tuberculosis Tuberculosis] where it is responsible for the persistence of ''Mycobacterium tuberculosis'' during the chronic stage of infection<ref name="genes">PMID: 18054522</ref> This survival strategy mediated by ICL is characterized by a metabolic shortcut within the [http://en.wikipedia.org/wiki/Citric_acid_cycle Citric Acid Cycle]. ICL creates this shunt pathway by converting isocitrate to succinate and glyoxylate, diverting acetyl-CoA from the beta-oxidation of fatty acids<ref name="ICL">PMID:10932251</ref><ref name="ICL2">PMID: 2696959</ref>. | ||
Revision as of 18:10, 19 April 2015
Isocitrate Lyase from Mycobacterium Tuberculosis
| |||||||||||
3D Structures of Isocitrate Lyase
Updated on 19-April-2015
- ICL from other bacteria
References
- ↑ Srivastava V, Jain A, Srivastava BS, Srivastava R. Selection of genes of Mycobacterium tuberculosis upregulated during residence in lungs of infected mice. Tuberculosis (Edinb). 2008 May;88(3):171-7. Epub 2007 Dec 3. PMID:18054522 doi:http://dx.doi.org/10.1016/j.tube.2007.10.002
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Sharma V, Sharma S, Hoener zu Bentrup K, McKinney JD, Russell DG, Jacobs WR Jr, Sacchettini JC. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat Struct Biol. 2000 Aug;7(8):663-8. PMID:10932251 doi:10.1038/77964
- ↑ 3.0 3.1 3.2 Beeching JR. High sequence conservation between isocitrate lyase from Escherichia coli and Ricinus communis. Protein Seq Data Anal. 1989 Dec;2(6):463-6. PMID:2696959
- ↑ 4.0 4.1 4.2 4.3 Masamune et al. Bio-Claisen condensation catalyzed by thiolase from Zoogloea ramigera. Active site cysteine residues. "Journal of the American Chemical Society" 111: 1879-1881 (1989). DOI: 10.1021/ja00187a053
- ↑ Connely, M. L. Solvent-accessible surfaces of proteins and nucleic acids "Science" 221:709-713 (1983). DOI: 10.1126/science.6879170