This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1087
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
== Introduction == | == Introduction == | ||
<StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | ||
| + | |||
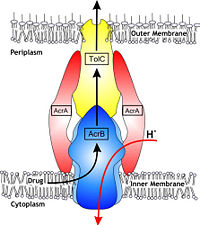
| + | [[Image:efflux pump.jpg|thumb|left|200px|Hypothetical structure of tripartite e¥ux pumps in Gram-negative bacteria, e.g. the AcrAB^TolC e¥ux pump from E. coli]] | ||
In Gram-negative bacteria, such as Escherichia coli, one of the main reasons behind bacterial multidrug resistance is resistance nodulation cell division (RND) transporters which pumps a wide range of antibiotics, dyes, bile salts and detergents out of the cell by proton motive force. Naturally, AcrB is speculated to function by pumping out of bile salts and their derivatives from the natural habitat of E. coli. for their survival with the presence of the high concentrations of these detergents. AcrB is the major RND transporter in E. coli. Three proteins form the tripartite multidrug efflux system that pumps the drugs out from the cell. The AcrB transporter, the inner membrane component of the system, cooperates with two other proteins: membrane fusion protein AcrA and an outer membrane channel TolC. The structure of the complex indicates that the drugs are transported out of the cell in a three-step procedure. [1, 2, 3] | In Gram-negative bacteria, such as Escherichia coli, one of the main reasons behind bacterial multidrug resistance is resistance nodulation cell division (RND) transporters which pumps a wide range of antibiotics, dyes, bile salts and detergents out of the cell by proton motive force. Naturally, AcrB is speculated to function by pumping out of bile salts and their derivatives from the natural habitat of E. coli. for their survival with the presence of the high concentrations of these detergents. AcrB is the major RND transporter in E. coli. Three proteins form the tripartite multidrug efflux system that pumps the drugs out from the cell. The AcrB transporter, the inner membrane component of the system, cooperates with two other proteins: membrane fusion protein AcrA and an outer membrane channel TolC. The structure of the complex indicates that the drugs are transported out of the cell in a three-step procedure. [1, 2, 3] | ||
| - | + | ||
This is a default text for your page ''''''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | This is a default text for your page ''''''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
| Line 16: | Line 18: | ||
The tripartite multidrug efflux system contains three components: AcrB, the inner membrane component, AcrA, the membrane fusion protein and TolC, the outer membrane channel. The TolC component is connected to outer membrane and AcrB is connected to the inner membrane. The AcrA connects TolC and AcrB together (Fig 1.). In the AcrB component there is three domains: TolC docking domain, pore domain and the transmembrane domain. The AcrB consists of 1 049 amino acids and the three protomers of AcrB are organized as a homotrimer (Fig. 2). The appearance of the trimer is of a jellyfish. The N- and C-terminal half’s show similar structural architecture and indicates at early gene duplication events. The trimer, formed by AcrB monomers, appears to be stabilized by the intermonomer connecting loops. [1, 2] | The tripartite multidrug efflux system contains three components: AcrB, the inner membrane component, AcrA, the membrane fusion protein and TolC, the outer membrane channel. The TolC component is connected to outer membrane and AcrB is connected to the inner membrane. The AcrA connects TolC and AcrB together (Fig 1.). In the AcrB component there is three domains: TolC docking domain, pore domain and the transmembrane domain. The AcrB consists of 1 049 amino acids and the three protomers of AcrB are organized as a homotrimer (Fig. 2). The appearance of the trimer is of a jellyfish. The N- and C-terminal half’s show similar structural architecture and indicates at early gene duplication events. The trimer, formed by AcrB monomers, appears to be stabilized by the intermonomer connecting loops. [1, 2] | ||
| - | + | [[Image:Nature01050-f2.2.jpg|thumb|right|350px|text]] | |
'''Transmembrane domain structure''' | '''Transmembrane domain structure''' | ||
Revision as of 10:11, 21 April 2015
| This Sandbox is Reserved from 15/04/2015, through 15/06/2015 for use in the course "Protein structure, function and folding" taught by Taru Meri at the University of Helsinki. This reservation includes Sandbox Reserved 1081 through Sandbox Reserved 1090. |
To get started:
More help: Help:Editing |
Introduction
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644