We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1063
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Introduction == | == Introduction == | ||
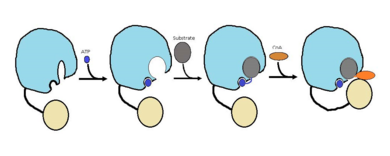
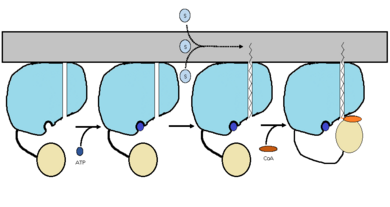
''<scene name='69/694230/Fadd13_subunits/5'>FadD13</scene>'' ''Mycobacterium Tuberculosis'' is an ACSVL (Acyl-CoA synthetases very long) peripheral membrane protein<ref>PMID: 17762044</ref>. ACS proteins activate [http://en.wikipedia.org/wiki/Lipid lipids] and [http://en.wikipedia.org/wiki/Fatty_acid fatty acids] before going into [http://en.wikipedia.org/wiki/Metabolic_pathway metabolic pathways]. FadD13 is soluble unlike other ACSVL proteins. FadD13 contains a hydrophobic tunnel for fatty acids to bind to, as well as an arginine rich lid loop that binds to the cell membrane. The binding of ATP causes structural changes promoting the binding of the hydrophobic substrates. Formation of an acyl-adenylate intermediate induces a 140 degree rotation of the small domain and binding of [http://en.wikipedia.org/wiki/Coenzyme_A CoA] for production of the final product, a fatty acyl-CoA thioester<ref>PMID: 19345228</ref>. Shown below is the general mechanism for ACS proteins. | ''<scene name='69/694230/Fadd13_subunits/5'>FadD13</scene>'' ''Mycobacterium Tuberculosis'' is an ACSVL (Acyl-CoA synthetases very long) peripheral membrane protein<ref>PMID: 17762044</ref>. ACS proteins activate [http://en.wikipedia.org/wiki/Lipid lipids] and [http://en.wikipedia.org/wiki/Fatty_acid fatty acids] before going into [http://en.wikipedia.org/wiki/Metabolic_pathway metabolic pathways]. FadD13 is soluble unlike other ACSVL proteins. FadD13 contains a hydrophobic tunnel for fatty acids to bind to, as well as an arginine rich lid loop that binds to the cell membrane. The binding of ATP causes structural changes promoting the binding of the hydrophobic substrates. Formation of an acyl-adenylate intermediate induces a 140 degree rotation of the small domain and binding of [http://en.wikipedia.org/wiki/Coenzyme_A CoA] for production of the final product, a fatty acyl-CoA thioester<ref>PMID: 19345228</ref>. Shown below is the general mechanism for ACS proteins. | ||
| - | [[Image:FadD13 steps.png|390 px|thumb|left|Figure 1 shows the general outline of the binding of ATP and acyl substrates to an ACSVL enzyme. This is the accepted mechanism for these types of proteins.]] | + | [[Image:FadD13 steps.png|390 px|thumb|left|Figure 1 shows the general outline of the binding of ATP and acyl substrates to an ACSVL enzyme. This is the accepted mechanism for these types of proteins.<ref name="Anderson 2012"/>]] |
== Background == | == Background == | ||
| Line 10: | Line 10: | ||
== Structural Highlights == | == Structural Highlights == | ||
| - | FadD13 is composed of 503 amino acids which are divided into two domains. The larger of the two domains is the N-terminal domain composed of <scene name='69/694230/Fadd13_subunits/8'>residues 1-395</scene> shown in blue. The smaller of the two domains is the C-terminal domain composed of <scene name='69/694230/Fadd13_subunits/10'>residues 402-503</scene> shown in yellow. These two domains are held together by a flexible 6 amino acid linker (<scene name='69/694230/Fadd13_subunits/11'>residues 396-401</scene>) shown in black. | + | FadD13 is composed of 503 amino acids which are divided into two domains. The larger of the two domains is the N-terminal domain composed of <scene name='69/694230/Fadd13_subunits/8'>residues 1-395</scene> shown in blue. The smaller of the two domains is the C-terminal domain composed of <scene name='69/694230/Fadd13_subunits/10'>residues 402-503</scene> shown in yellow. These two domains are held together by a flexible 6 amino acid linker (<scene name='69/694230/Fadd13_subunits/11'>residues 396-401</scene>) shown in black.<ref name="Anderson 2012"/> |
===Active Site=== | ===Active Site=== | ||
The active site of FadD13 is composed of an <scene name='69/694230/Fadd13_subunits/12'>ATP/AMP binding region</scene>. This region is comprised of residues 164-TSGTTGHPKG173-173 shown in red which binds to the phosphate group, and residues 298-VQGYALTE-305 shown in blue which binds to the adenine group<ref name="Khare 2009">DOI: 10.1371/journal.pone.0008387</ref>. Upon binding of ATP/AMP, FadD13 becomes activated and allows for binding of lipids or fatty acids. | The active site of FadD13 is composed of an <scene name='69/694230/Fadd13_subunits/12'>ATP/AMP binding region</scene>. This region is comprised of residues 164-TSGTTGHPKG173-173 shown in red which binds to the phosphate group, and residues 298-VQGYALTE-305 shown in blue which binds to the adenine group<ref name="Khare 2009">DOI: 10.1371/journal.pone.0008387</ref>. Upon binding of ATP/AMP, FadD13 becomes activated and allows for binding of lipids or fatty acids. | ||
Revision as of 17:52, 21 April 2015
FadD13
| |||||||||||
References
- ↑ Watkins PA, Maiguel D, Jia Z, Pevsner J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J Lipid Res. 2007 Dec;48(12):2736-50. Epub 2007 Aug 30. PMID:17762044 doi:http://dx.doi.org/M700378-JLR200
- ↑ Kochan G, Pilka ES, von Delft F, Oppermann U, Yue WW. Structural snapshots for the conformation-dependent catalysis by human medium-chain acyl-coenzyme A synthetase ACSM2A. J Mol Biol. 2009 May 22;388(5):997-1008. Epub 2009 Apr 1. PMID:19345228 doi:10.1016/j.jmb.2009.03.064
- ↑ 3.0 3.1 3.2 3.3 3.4 Andersson CS, Lundgren CA, Magnusdottir A, Ge C, Wieslander A, Molina DM, Hogbom M. The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: Structural Basis for Housing Lipid Substrates Longer than the Enzyme. Structure. 2012 May 2. PMID:22560731 doi:10.1016/j.str.2012.03.012
- ↑ 4.0 4.1 Khare G, Gupta V, Gupta RK, Gupta R, Bhat R, Tyagi AK. Dissecting the role of critical residues and substrate preference of a Fatty Acyl-CoA Synthetase (FadD13) of Mycobacterium tuberculosis. PLoS One. 2009 Dec 21;4(12):e8387. doi: 10.1371/journal.pone.0008387. PMID:20027301 doi:10.1371/journal.pone.0008387