Sandbox Reserved 1058
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
===Structure=== | ===Structure=== | ||
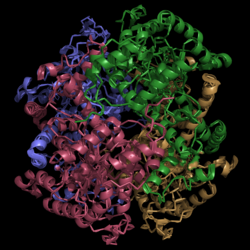
[[Image:Normal_Crystal_Structure.png|250 px|left|thumb|'''Figure 1. Crystal Structure of Isocitrate Lyase.''' Quaternary structure is comprised of four subunits forming an α/β barrel. A side view is shown where each comprising subunit is a different color with the central hole of the barrel coming perpendicularly out of the page.]] | [[Image:Normal_Crystal_Structure.png|250 px|left|thumb|'''Figure 1. Crystal Structure of Isocitrate Lyase.''' Quaternary structure is comprised of four subunits forming an α/β barrel. A side view is shown where each comprising subunit is a different color with the central hole of the barrel coming perpendicularly out of the page.]] | ||
| - | <scene name='69/694225/Isocitrate_lyase/ | + | <scene name='69/694225/Isocitrate_lyase/3'>Isocitrate lyase</scene> is a tetramer with 222 symmetry. Each <scene name='69/694225/Subunit_a/2'>subunit</scene> is composed of 14 alpha helices and 14 beta sheets which includes a total of 426 residues. These α helices and β sheets form an unusual <scene name='69/694225/Isocitrate_lyase/2'>α/β barrel</scene> seen in '''Figure 1'''. The α/β barrel contains a topology of (βα)<sub>2</sub>α(βα)<sub>5</sub>β, differing from the canonical (βα)<sub>8</sub> pattern. Residues 184-200 and 235-254 connects the third and forth β-strands to their consecutive helices and form a <scene name='69/694225/Small_beta_domain/1'>small β-domain</scene> that consists of a short five-stranded βsheet (β6,β7,β9,β10,β11) that lies on top of the α/β barrel in the orientation shown in '''Figure 1'''. <ref name="sharma"> Sharma, V.; Sharma, S.; Hoener zu Bentrup, K.; McKinney, J.; Russell, D.; ''et. al''; Structure of isocitrate lyase, a persistence factor of ''Mycobacterium tuberculosis''. ''Nat. Struct. Biol.''. '''2000'''. ''7(8)'':663-668. </ref> Additionally, this β-domain contains the catalytic loop necessary for isocitrate lyase to breakdown isocitrate. A study of the equilibria between the <scene name='69/694225/Isocitrate_lyase/3'>four subunits</scene> shows that each isocitrate lyase monomer has a dynamic comformational change of the active site loop. At any given time, only two of the subunits are in the open conformation. <ref name="gould"> Gould, T.; van de Langemheen, H.; Muñoz-Elías, E.; McKinney, D.; Sacchettini, J.; Dual role of isocitrate lyase 1 in the glyoxylate and methylcitrate cycles in ''Mycobacterium tuberculosis''. ''Molecular Microbiology''. '''2006'''. ''61(4)'':940-947. doi:10.1111/j.1365-2958.2006.05297.x. </ref> Furthermore, isocitrate lyase shows a resemblance to [http://www.rcsb.org/pdb/explore/explore.do?structureId=1S2V phosphoenolpyrvate mutase]. <ref name="sharma"> Sharma, V.; Sharma, S.; Hoener zu Bentrup, K.; McKinney, J.; Russell, D.; ''et. al''; Structure of isocitrate lyase, a persistence factor of ''Mycobacterium tuberculosis''. ''Nat. Struct. Biol.''. '''2000'''. ''7(8)'':663-668. </ref> |
| Line 23: | Line 23: | ||
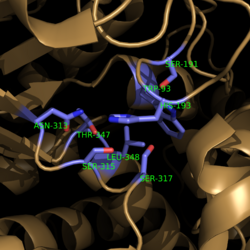
===Catalytic Loop=== | ===Catalytic Loop=== | ||
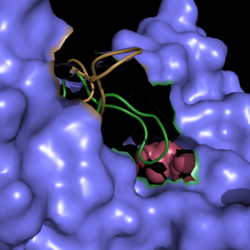
| - | [[Image:Active Loop Shift.png|250 px|left|thumb|'''Figure 4. Active Site Loop Shift.''' Binding of the ligand to the enzyme results in a conformational shift that facilitates the breakdown of isocitrate. The active site loop without a ligand bound is shown in wheat while the active site loop with a ligand bound is shown in green. The ligands are shown in raspberry.]] The <scene name='69/694225/Normal_catalytic_loop/1'>catalytic loop</scene> of isocitrate lyase consists of residues 185-196 ('''Figure 4'''). The two most important residues within the loop are <scene name='69/694225/Normal_catalytic_loop/ | + | [[Image:Active Loop Shift.png|250 px|left|thumb|'''Figure 4. Active Site Loop Shift.''' Binding of the ligand to the enzyme results in a conformational shift that facilitates the breakdown of isocitrate. The active site loop without a ligand bound is shown in wheat while the active site loop with a ligand bound is shown in green. The ligands are shown in raspberry.]] The <scene name='69/694225/Normal_catalytic_loop/1'>catalytic loop</scene> of isocitrate lyase consists of residues 185-196 ('''Figure 4'''). The two most important residues within the loop are <scene name='69/694225/Normal_catalytic_loop/3'>Cys191 and His193</scene> as these form a charge relay strong enough to extract a proton from isocitrate. Poor electron density has been observed for residues His193 and Leu194 indicating that this loop is very flexible. <ref name="sharma"> Sharma, V.; Sharma, S.; Hoener zu Bentrup, K.; McKinney, J.; Russell, D.; ''et. al''; Structure of isocitrate lyase, a persistence factor of ''Mycobacterium tuberculosis''. ''Nat. Struct. Biol.''. '''2000'''. ''7(8)'':663-668. </ref> This data backs up the claim that that monomers of the protein are in a structural equilibria between the open and closed forms of the active site. In order for the catalytic loop to shift into the closed position necessary for catalysis, isocitrate must be within the binding pocket. The hydrogen bonding opportunities formed cause a ripple effect that shifts the catalytic loop into a closer position. <ref name="sharma"> Sharma, V.; Sharma, S.; Hoener zu Bentrup, K.; McKinney, J.; Russell, D.; ''et. al''; Structure of isocitrate lyase, a persistence factor of ''Mycobacterium tuberculosis''. ''Nat. Struct. Biol.''. '''2000'''. ''7(8)'':663-668. </ref> This shift also causes the C-terminal domain (blue) of the subunit (residues 411-428) to <scene name='69/694225/C-terminus_loop_in_cat_loop/4'>move</scene> into the former position of the catalytic loop (green). Also shown as a reference is the ligand (pink). The C-terminal domain is then stabilized by an <scene name='69/694225/Lys_electrostatic/3'>electrostatic interaction</scene> with Lys189. This combined movement locks the active site residues into a proper orientation for lysis of a C-C bond within isocitrate. <ref name="sharma"> Sharma, V.; Sharma, S.; Hoener zu Bentrup, K.; McKinney, J.; Russell, D.; ''et. al''; Structure of isocitrate lyase, a persistence factor of ''Mycobacterium tuberculosis''. ''Nat. Struct. Biol.''. '''2000'''. ''7(8)'':663-668. </ref> |
===Regulation=== | ===Regulation=== | ||
| - | <scene name='69/694225/Isocitrate_lyase/ | + | <scene name='69/694225/Isocitrate_lyase/3'>Isocitrate lyase</scene> competes with [http://en.wikipedia.org/wiki/Isocitrate_dehydrogenase isocitrate dehydrogenase], an enzyme found in the [http://en.wikipedia.org/wiki/Citric_acid_cycle citric cycle], for isocitrate processing. The favoritism of one enzyme over the other is controlled by the phosphorylation of isocitrate dehydrogenase. This enzyme has a much higher affinity for isocitrate as compared to isocitrate lyase. Phosphorylation of isocitrate dehydrogenase inactivates the enzyme and leades to increased isocitrate lyase activity. <ref name="cozzone"> Cozzone, A.; Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. ''Annual Review of Microbiology''. '''1998''', ''52'':127-164. doi: 10.1146/annurev.micro.52.1.127. </ref> |
| Line 34: | Line 34: | ||
==Mechanism of Action== | ==Mechanism of Action== | ||
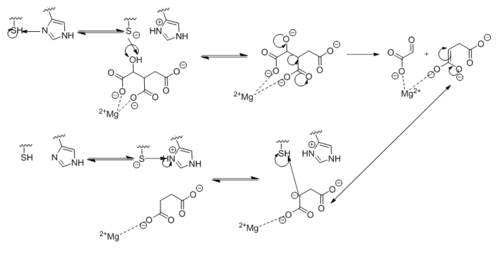
[[Image:Complete_Mechanism.PNG|500 px|right|thumb|'''Figure 5. Observed Mechanism for the Breakdown of Isocitrate by Isocitrate Lyase.''']] | [[Image:Complete_Mechanism.PNG|500 px|right|thumb|'''Figure 5. Observed Mechanism for the Breakdown of Isocitrate by Isocitrate Lyase.''']] | ||
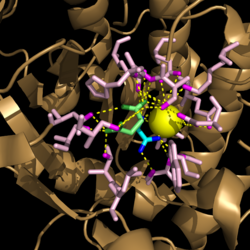
| - | Within <scene name='69/694225/Pka_shift/ | + | Within <scene name='69/694225/Pka_shift/2'>isocitrate lyase</scene>, His193 shifts the pKa of Cys191 and removes its proton. This allows Cys191 to extract a proton from the hydroxyl group of isocitrate. The resulting oxyanion forms a carbonyl and forces the lysis of a C-C bond. Glyoxylate and the enol form of succinate are formed and stabilized with a Mg<sup>2+</sup> ion. The succinate enolate resonates and extracts the proton back from Cys191 to form succinate ('''Figure 5'''). |
| Line 59: | Line 59: | ||
===Clinical Implications=== | ===Clinical Implications=== | ||
[[Image:TCA_Cycle.png|500 px|left|thumb|'''Figure 6. Citric Acid Cycle with Glyoxylate Shunt Pathway.''' In several bacterial species, there is a carbon conserving gloxylate shunt pathway that converts isocitrate to malate in two steps instead of the usual five steps.]] ''Mycobacterium tuberculosis'' is a respiratory infection that causes numerous fatalities throughout the world. It lives in organisms and feeds off of host cells, which indicate a variety of lipases exist within ''M. tuberculosis''. Current drugs that are on the market now target a small number of bacterial processes like cell wall formation and chromosomal replication. Although several antibiotics exist, all of them target these same mechanisms of inhibition. These commonalities have led to the prevalence of different multi-drug resistant (MDR) tuberculosis strains. Due to the high level of resistance, finding a lasting treatment for MDR TB infections has become very problematic. Studies into new mechanisms of inhibition will be crucial to prevent widespread outbreaks. | [[Image:TCA_Cycle.png|500 px|left|thumb|'''Figure 6. Citric Acid Cycle with Glyoxylate Shunt Pathway.''' In several bacterial species, there is a carbon conserving gloxylate shunt pathway that converts isocitrate to malate in two steps instead of the usual five steps.]] ''Mycobacterium tuberculosis'' is a respiratory infection that causes numerous fatalities throughout the world. It lives in organisms and feeds off of host cells, which indicate a variety of lipases exist within ''M. tuberculosis''. Current drugs that are on the market now target a small number of bacterial processes like cell wall formation and chromosomal replication. Although several antibiotics exist, all of them target these same mechanisms of inhibition. These commonalities have led to the prevalence of different multi-drug resistant (MDR) tuberculosis strains. Due to the high level of resistance, finding a lasting treatment for MDR TB infections has become very problematic. Studies into new mechanisms of inhibition will be crucial to prevent widespread outbreaks. | ||
| - | <scene name='69/694225/Isocitrate_lyase/ | + | <scene name='69/694225/Isocitrate_lyase/4'>Isocitrate lyase</scene> plays a key role in survival of ''M. tuberculosis'' by sustaining intracellular infections in inflammatory respiratory macrophages.<ref name="muñoz-elías"> Muñoz-Elías, E.; McKinney, J.; ''M. tuberculosis'' isocitrate lyases 1 and 2 are jointly required for ''in vivo'' growth and virulence. ''Nat. Med.'' '''2005'''. ''11(6)'':638-644. doi:10.1038/nm1252. </ref> Used in the citric acid cycle, isocitrate lyase is the first enzyme catalyzing the carbon conserving glyoxylate pathway ('''Figure 6'''). This glyoxylate pathway has not been observed in mammals and thus presents a unique drug target to solely attack TB infections. Research has shown that upregulation of the glyoxylate cycle occurs for pathogens like ''M. tuberculosis'' during an infection. <ref name="srivastava"> Srivastava, V.; Janin, A.; Srivastava, B.; Srivastava, R.; Selection of genes of ''Mycobacterium tuberculosis'' upregulated during residence in lungs of infected mice. ''ScienceDirect''. '''2007'''. doi:10.1016/j.tube.2007.10.002. </ref> |
Revision as of 17:22, 24 April 2015
Isocitrate Lyase from Mycobacterium tuberculosis
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Sharma, V.; Sharma, S.; Hoener zu Bentrup, K.; McKinney, J.; Russell, D.; et. al; Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat. Struct. Biol.. 2000. 7(8):663-668.
- ↑ Gould, T.; van de Langemheen, H.; Muñoz-Elías, E.; McKinney, D.; Sacchettini, J.; Dual role of isocitrate lyase 1 in the glyoxylate and methylcitrate cycles in Mycobacterium tuberculosis. Molecular Microbiology. 2006. 61(4):940-947. doi:10.1111/j.1365-2958.2006.05297.x.
- ↑ Cozzone, A.; Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annual Review of Microbiology. 1998, 52:127-164. doi: 10.1146/annurev.micro.52.1.127.
- ↑ Muñoz-Elías, E.; McKinney, J.; M. tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 2005. 11(6):638-644. doi:10.1038/nm1252.
- ↑ Srivastava, V.; Janin, A.; Srivastava, B.; Srivastava, R.; Selection of genes of Mycobacterium tuberculosis upregulated during residence in lungs of infected mice. ScienceDirect. 2007. doi:10.1016/j.tube.2007.10.002.
- ↑ Dunn, M.; Ramírez-Trujillo, J.; Hernández-Lucas, I.; Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology. 2009. 155:3166-3175. doi:10.1099/mic.0.030858-0.