We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1058
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
===Structure=== | ===Structure=== | ||
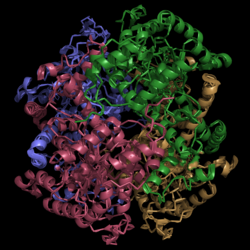
[[Image:Normal_Crystal_Structure.png|250 px|left|thumb|'''Figure 1. Crystal Structure of Isocitrate Lyase.''' Quaternary structure is comprised of four subunits forming an α/β barrel. A side view is shown where each comprising subunit is a different color with the central hole of the barrel coming perpendicularly out of the page.]] | [[Image:Normal_Crystal_Structure.png|250 px|left|thumb|'''Figure 1. Crystal Structure of Isocitrate Lyase.''' Quaternary structure is comprised of four subunits forming an α/β barrel. A side view is shown where each comprising subunit is a different color with the central hole of the barrel coming perpendicularly out of the page.]] | ||
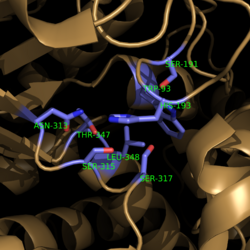
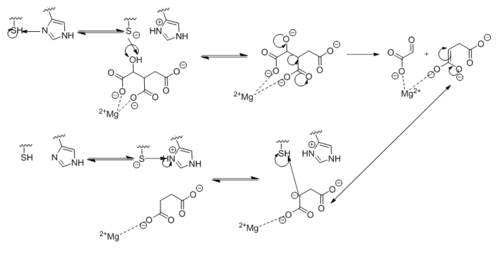
| - | <scene name='69/694225/Isocitrate_lyase/4'>Isocitrate lyase</scene> is a tetramer with 222 symmetry. Each <scene name='69/694225/Subunit_a/2'>subunit</scene> is composed of 14 alpha helices and 14 beta sheets which includes a total of 426 residues. These α helices and β sheets form an unusual <scene name='69/694225/ | + | <scene name='69/694225/Isocitrate_lyase/4'>Isocitrate lyase</scene> is a tetramer with 222 symmetry. Each <scene name='69/694225/Subunit_a/2'>subunit</scene> is composed of 14 alpha helices and 14 beta sheets which includes a total of 426 residues. These α helices and β sheets form an unusual <scene name='69/694225/Beta_barrel/1'>α/β barrel</scene>. The α/β barrel contains a topology of (βα)<sub>2</sub>α(βα)<sub>5</sub>β, differing from the canonical (βα)<sub>8</sub> pattern. Residues 184-200 and 235-254 connects the third and forth β-strands to their consecutive helices and form a <scene name='69/694225/Beta_domain/1'>small β-domain</scene> that consists of a short five-stranded βsheet (β6,β7,β9,β10,β11) that lies on top of the α/β barrel. <ref name="sharma"> Sharma, V.; Sharma, S.; Hoener zu Bentrup, K.; McKinney, J.; Russell, D.; ''et. al''; Structure of isocitrate lyase, a persistence factor of ''Mycobacterium tuberculosis''. ''Nat. Struct. Biol.''. '''2000'''. ''7(8)'':663-668. </ref> Additionally, this β-domain contains the catalytic loop necessary for isocitrate lyase to breakdown isocitrate. A study of the equilibria between the <scene name='69/694225/Isocitrate_lyase/4'>four subunits</scene> shows that each isocitrate lyase monomer has a dynamic comformational change of the active site loop. At any given time, only two of the subunits are in the open conformation. <ref name="gould"> Gould, T.; van de Langemheen, H.; Muñoz-Elías, E.; McKinney, D.; Sacchettini, J.; Dual role of isocitrate lyase 1 in the glyoxylate and methylcitrate cycles in ''Mycobacterium tuberculosis''. ''Molecular Microbiology''. '''2006'''. ''61(4)'':940-947. doi:10.1111/j.1365-2958.2006.05297.x. </ref> Furthermore, isocitrate lyase shows a resemblance to [http://www.rcsb.org/pdb/explore/explore.do?structureId=1S2V phosphoenolpyrvate mutase]. <ref name="sharma"> Sharma, V.; Sharma, S.; Hoener zu Bentrup, K.; McKinney, J.; Russell, D.; ''et. al''; Structure of isocitrate lyase, a persistence factor of ''Mycobacterium tuberculosis''. ''Nat. Struct. Biol.''. '''2000'''. ''7(8)'':663-668. </ref> |
Revision as of 18:22, 24 April 2015
Isocitrate Lyase from Mycobacterium tuberculosis
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Sharma, V.; Sharma, S.; Hoener zu Bentrup, K.; McKinney, J.; Russell, D.; et. al; Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat. Struct. Biol.. 2000. 7(8):663-668.
- ↑ Gould, T.; van de Langemheen, H.; Muñoz-Elías, E.; McKinney, D.; Sacchettini, J.; Dual role of isocitrate lyase 1 in the glyoxylate and methylcitrate cycles in Mycobacterium tuberculosis. Molecular Microbiology. 2006. 61(4):940-947. doi:10.1111/j.1365-2958.2006.05297.x.
- ↑ Cozzone, A.; Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annual Review of Microbiology. 1998, 52:127-164. doi: 10.1146/annurev.micro.52.1.127.
- ↑ Muñoz-Elías, E.; McKinney, J.; M. tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 2005. 11(6):638-644. doi:10.1038/nm1252.
- ↑ Srivastava, V.; Janin, A.; Srivastava, B.; Srivastava, R.; Selection of genes of Mycobacterium tuberculosis upregulated during residence in lungs of infected mice. ScienceDirect. 2007. doi:10.1016/j.tube.2007.10.002.
- ↑ Dunn, M.; Ramírez-Trujillo, J.; Hernández-Lucas, I.; Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology. 2009. 155:3166-3175. doi:10.1099/mic.0.030858-0.