Sandbox Reserved 1059
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='4K8M' size='350' side='right' caption='NrdH of ''Mycobacterium tuberculosis''' (PDB entry [[4K8M]])' scene=''> | <StructureSection load='4K8M' size='350' side='right' caption='NrdH of ''Mycobacterium tuberculosis''' (PDB entry [[4K8M]])' scene=''> | ||
==='''Introduction'''=== | ==='''Introduction'''=== | ||
| - | NrdH | + | NrdH is a redox protein, and it is part of a family of redox proteins. The other proteins that maintain the redox balance of NrdH are three [http://en.wikipedia.org/wiki/Thioredoxin thioredoxin] and three [http://en.wikipedia.org/wiki/Glutaredoxin glutaredoxins]-like proteins. Prokaryotes typically maintain redox homeostasis through low-molecular weight thiols (glutathione) and through proteins involved in disulfide exchange (thioredoxins). NrdH is found in many types of bacteria, such as [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis ''Mycobacterium tuberculosis'']. This bacteria causes the disease [http://en.wikipedia.org/wiki/Tuberculosis tuberculosis], which is a fatal disease if not treated properly. Tuberculosis was the leading cause of death in the United States in the past, and it can be spread through the air from one person to another by coughing, sneezing, or speaking <ref>DOI: 10.1016/j.tube.2003.08.003</ref>. |
| + | |||
| + | [[Image:Image_2_(2).png|350px|left|thumb|Aromatic amino acids binding site]] | ||
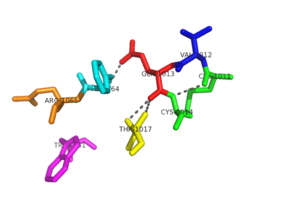
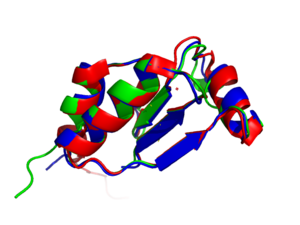
| + | [[Image:Ligands.png|300px|left|thumb|Basic structure of NrdH. Contains two bound ligands, three alpha helices, and four beta sheets. ]] | ||
| + | The tertiary structure of NrdH has a thioredoxin fold with 79 residues with a glutaredoxin-like sequence. However, unlike glutaredoxins, NrdH of ''[[Mycobacterium tuberculosis]]'' can accept electrons from thioredoxin reductase. The binding site of NrdH is specific for aromatic amino acids <ref name="Phulera" />. | ||
| + | |||
| + | ==Function== | ||
| + | NrdH redoxins are small reductases, and they contain similar amino acid sequences to glutaredoxins and [http://en.wikipedia.org/wiki/Mycoredoxin mycoredoxins]. However, NrdH redoxins are different because of their thioredoxin-like activity. Their main function is to act as the electron donor for class 1b [http://en.wikipedia.org/wiki/Ribonucleotide_reductase ribonucleotide reductases], which are important for the conversion of [http://en.wikipedia.org/wiki/Ribonucleotide ribonucleotides] to [http://en.wikipedia.org/wiki/Deoxyribonucleotide deoxyribonucleotides] <ref name="Laer">DOI: 10.1074/jbc.M112.392688</ref>. The process of ribonucleotide reduction is one of the most fundamental biochemical processes that is required for the existence of DNA-based life. It is the only de novo pathway to synthesize deoxyribonucleotides. Deoxyribonucleotides are the building blocks of DNA and are synthesized from ribonucleotides by reducing the 2’OH in a radical based reaction. The deoxyribonucleotides are then used as precursors for the process of DNA synthesis. This reaction is catalyzed by ribonucleotide reductases <ref name="Phulera">DOI: 10.1021/bi400191z</ref>. | ||
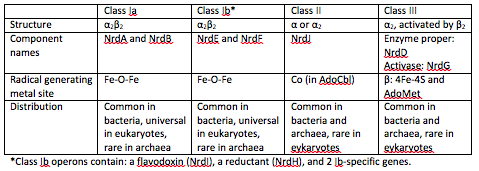
==='''Ribonucleotide Reductases (RNRs)'''=== | ==='''Ribonucleotide Reductases (RNRs)'''=== | ||
| Line 17: | Line 24: | ||
At the end of each cycle of ribonucleotide reduction, the ribonucleotide reductase needs to be reduced in order to be ready for the next reduction cycle. For a class 1a RNR, an external cofactor, such as a glutaredoxin or thioredoxin, performs this reduction step. For class 1b RNRs, this cofactor is known as NrdH. NrdH contains a glutaredoxin-like sequence but behaves like a thioredoxin <ref name="Phulera" />. | At the end of each cycle of ribonucleotide reduction, the ribonucleotide reductase needs to be reduced in order to be ready for the next reduction cycle. For a class 1a RNR, an external cofactor, such as a glutaredoxin or thioredoxin, performs this reduction step. For class 1b RNRs, this cofactor is known as NrdH. NrdH contains a glutaredoxin-like sequence but behaves like a thioredoxin <ref name="Phulera" />. | ||
| - | == | + | == Structure == |
| - | |||
| - | NrdH is a redox protein, and it is part of a family of redox proteins. The other proteins that maintain the redox balance of NrdH are three thioredoxin and three glutaredoxin-like proteins. Prokaryotes typically maintain redox homeostasis through low-molecular weight thiols (glutathione) and through proteins involved in disulfide exchange (thioredoxins). | ||
| - | |||
| - | [[Image:Image_2_(2).png|350px|left|thumb|Aromatic amino acids binding site]] | ||
| - | |||
| - | NrdH is found in many types of bacteria, such as [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis ''Mycobacterium tuberculosis'']. This bacteria causes the disease [http://en.wikipedia.org/wiki/Tuberculosis tuberculosis], which is a fatal disease if not treated properly. Tuberculosis was the leading cause of death in the United States in the past, and it can be spread through the air from one person to another by coughing, sneezing, or speaking <ref>DOI: 10.1016/j.tube.2003.08.003</ref>. | ||
| - | |||
| - | == Structure == | ||
| - | [[Image:Ligands.png|300px|left|thumb|Basic structure of NrdH. Contains two bound ligands, three alpha helices, and four beta sheets. ]] | ||
| - | The tertiary structure of NrdH has a thioredoxin fold with 79 residues with a glutaredoxin-like sequence. However, unlike glutaredoxins, NrdH of ''[[Mycobacterium tuberculosis]]'' can accept electrons from thioredoxin reductase. The binding site of NrdH is specific for aromatic amino acids <ref name="Phulera" />. | ||
===Conserved Motifs=== | ===Conserved Motifs=== | ||
Revision as of 17:08, 25 April 2015
NrdH of Mycobacterium tuberculosis
| |||||||||||