We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1059
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | == | + | ==NrdH of ''Mycobacterium tuberculosis''== |
<StructureSection load='4K8M' size='350' side='right' caption='NrdH of ''Mycobacterium tuberculosis''' (PDB entry [[4K8M]])' scene=''> | <StructureSection load='4K8M' size='350' side='right' caption='NrdH of ''Mycobacterium tuberculosis''' (PDB entry [[4K8M]])' scene=''> | ||
| - | == | + | ==Introduction== |
NrdH is a redox protein and is part of a family of redox proteins. The other proteins that maintain the redox balance of NrdH are three [http://en.wikipedia.org/wiki/Thioredoxin thioredoxin] and three [http://en.wikipedia.org/wiki/Glutaredoxin glutaredoxins]-like proteins. Prokaryotes typically maintain redox homeostasis through low-molecular weight thiols (glutathione) and through proteins involved in disulfide exchange (thioredoxins)<ref name="Phulera" />. NrdH is found in many types of bacteria, such as [http://www.nature.com/nature/journal/v393/n6685/full/393537a0.html ''Mycobacterium tuberculosis'']. This bacteria causes the disease [http://en.wikipedia.org/wiki/Tuberculosis tuberculosis] <ref name="Cole"> DOI: 10.1038/31159</ref>. | NrdH is a redox protein and is part of a family of redox proteins. The other proteins that maintain the redox balance of NrdH are three [http://en.wikipedia.org/wiki/Thioredoxin thioredoxin] and three [http://en.wikipedia.org/wiki/Glutaredoxin glutaredoxins]-like proteins. Prokaryotes typically maintain redox homeostasis through low-molecular weight thiols (glutathione) and through proteins involved in disulfide exchange (thioredoxins)<ref name="Phulera" />. NrdH is found in many types of bacteria, such as [http://www.nature.com/nature/journal/v393/n6685/full/393537a0.html ''Mycobacterium tuberculosis'']. This bacteria causes the disease [http://en.wikipedia.org/wiki/Tuberculosis tuberculosis] <ref name="Cole"> DOI: 10.1038/31159</ref>. | ||
| Line 8: | Line 8: | ||
Tuberculosis (TB) is a contagious, fatal disease if not treated properly. It affects the lungs mostly, but can have detrimental affects on other organs of the body as well. TB bacteria can be latent and live inside the body in a dormant state without causing any symptoms. When it becomes active, it results in symptoms of bad coughing, chest pain, and others. It is typically treated with several drugs taken for 6-9 months.It was the leading cause of death in the United States in the past, and it can be spread through the air from one person to another by coughing, sneezing, or speaking <ref>Tuberculosis (TB). ''PubMed Health''. Retrieved from http://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0024668/</ref>. | Tuberculosis (TB) is a contagious, fatal disease if not treated properly. It affects the lungs mostly, but can have detrimental affects on other organs of the body as well. TB bacteria can be latent and live inside the body in a dormant state without causing any symptoms. When it becomes active, it results in symptoms of bad coughing, chest pain, and others. It is typically treated with several drugs taken for 6-9 months.It was the leading cause of death in the United States in the past, and it can be spread through the air from one person to another by coughing, sneezing, or speaking <ref>Tuberculosis (TB). ''PubMed Health''. Retrieved from http://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0024668/</ref>. | ||
| - | == | + | ==Structure== |
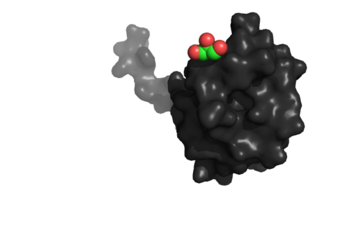
[[Image:Image_2_(2).png|350px|left|thumb|Binding site specific for aromatic amino acids. The hole that is located in the center of this image of NrdH shows the binding site.]] | [[Image:Image_2_(2).png|350px|left|thumb|Binding site specific for aromatic amino acids. The hole that is located in the center of this image of NrdH shows the binding site.]] | ||
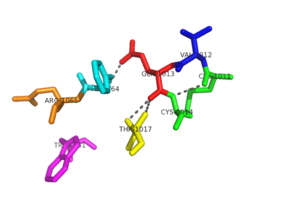
The <scene name='69/694226/Tertiary_structure/2'>tertiary structure</scene> of NrdH has a thioredoxin fold with 79 residues with a glutaredoxin-like sequence. Unlike glutaredoxins, NrdH of ''Mycobacterium tuberculosis'' can accept electrons from thioredoxin reductase<ref name="Phulera" />. It contains two bound ligands, three alpha helices, and four beta sheets. The two ligands are colored by element, red representing oxygen and gray representing carbon. The binding site of NrdH is specific for aromatic amino acids. The image on the left shows the specific binding site. The specificity for aromatic amino acids is vital because aromatic-aromatic interactions have shown to have great importance for protein folding, ligand binding, and protein stability <ref name="Lanzarotti">DOI: 10.1021/ci200062e</ref>. | The <scene name='69/694226/Tertiary_structure/2'>tertiary structure</scene> of NrdH has a thioredoxin fold with 79 residues with a glutaredoxin-like sequence. Unlike glutaredoxins, NrdH of ''Mycobacterium tuberculosis'' can accept electrons from thioredoxin reductase<ref name="Phulera" />. It contains two bound ligands, three alpha helices, and four beta sheets. The two ligands are colored by element, red representing oxygen and gray representing carbon. The binding site of NrdH is specific for aromatic amino acids. The image on the left shows the specific binding site. The specificity for aromatic amino acids is vital because aromatic-aromatic interactions have shown to have great importance for protein folding, ligand binding, and protein stability <ref name="Lanzarotti">DOI: 10.1021/ci200062e</ref>. | ||
| Line 27: | Line 27: | ||
A buried water molecule binds with the WSGFRP motif. This water is believed to be one of the structural signatures of NrdH proteins. The hydrogen bonding network of the CVQC and WSGFRP motifs also involves the water molecule, and this may suggest that this region is important in the evolution of NrdH <ref name="Phulera" />. | A buried water molecule binds with the WSGFRP motif. This water is believed to be one of the structural signatures of NrdH proteins. The hydrogen bonding network of the CVQC and WSGFRP motifs also involves the water molecule, and this may suggest that this region is important in the evolution of NrdH <ref name="Phulera" />. | ||
| - | == | + | ==Function== |
The main function of NrdH is to act as a reducing partner of class 1B ribonucleotide reductase and for ribonucleotide reduction (RR) and is thought to supply electrons for this biochemical reaction. RR is one of the most fundamental biochemical processes that is required for DNA based life form to exist. Ribonucleotide reductases (RNRs) produce deoxyribonucleotides, which are precursors for DNA synthesis. NrdH is able to accept electrons from ''M. tuberculosis'' thioredoxin reductase and is able to reduce the disulfide bonds that are present in insulin <ref name="Phulera" />. | The main function of NrdH is to act as a reducing partner of class 1B ribonucleotide reductase and for ribonucleotide reduction (RR) and is thought to supply electrons for this biochemical reaction. RR is one of the most fundamental biochemical processes that is required for DNA based life form to exist. Ribonucleotide reductases (RNRs) produce deoxyribonucleotides, which are precursors for DNA synthesis. NrdH is able to accept electrons from ''M. tuberculosis'' thioredoxin reductase and is able to reduce the disulfide bonds that are present in insulin <ref name="Phulera" />. | ||
| Line 44: | Line 44: | ||
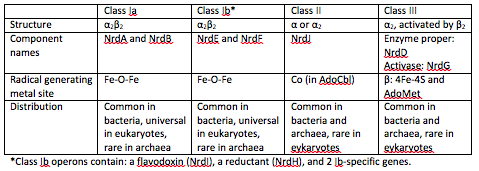
At the end of each cycle of ribonucleotide reduction, the ribonucleotide reductase needs to be reduced in order to be ready for the next reduction cycle. For a class 1a RNR, an external cofactor, such as a glutaredoxin or thioredoxin, performs this reduction step. For class 1b RNRs, this cofactor is known as NrdH. NrdH contains a glutaredoxin-like sequence but behaves like a thioredoxin <ref name="Phulera" />. | At the end of each cycle of ribonucleotide reduction, the ribonucleotide reductase needs to be reduced in order to be ready for the next reduction cycle. For a class 1a RNR, an external cofactor, such as a glutaredoxin or thioredoxin, performs this reduction step. For class 1b RNRs, this cofactor is known as NrdH. NrdH contains a glutaredoxin-like sequence but behaves like a thioredoxin <ref name="Phulera" />. | ||
| - | == | + | ==Drug Targets== |
[[Image:Image_7_(1).png|300px|right|thumb|Sequence alignment of NrdH from ''Mycobacterium tuberculosis'', ''Corynebacterium glutamicum'', and ''Echerichia coli'']] | [[Image:Image_7_(1).png|300px|right|thumb|Sequence alignment of NrdH from ''Mycobacterium tuberculosis'', ''Corynebacterium glutamicum'', and ''Echerichia coli'']] | ||
Genes that encode for NrdE and NrdF are essential for growth, and RR might be an attractive biochemical pathway for antimycobacterial drug discovery. Organisms that depend solely on class 1B RNR could potentially contain the essential genes and serve as potential drug targets for treating tuberculosis <ref name="Phulera" />. | Genes that encode for NrdE and NrdF are essential for growth, and RR might be an attractive biochemical pathway for antimycobacterial drug discovery. Organisms that depend solely on class 1B RNR could potentially contain the essential genes and serve as potential drug targets for treating tuberculosis <ref name="Phulera" />. | ||
| - | == | + | ==References== |
<references/> | <references/> | ||
</StructureSection> | </StructureSection> | ||
Revision as of 14:58, 26 April 2015
NrdH of Mycobacterium tuberculosis
| |||||||||||