Sandbox Reserved 1068
From Proteopedia
| Line 1: | Line 1: | ||
==''Mycobacterium tuberculosis'' salicylate synthase (Mbt1)== | ==''Mycobacterium tuberculosis'' salicylate synthase (Mbt1)== | ||
<StructureSection load='3LOG' size='450' side='right' caption='([[3LOG]]) is a 4 chain structure of MbtI with sequence from [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3LOG OCA].'> | <StructureSection load='3LOG' size='450' side='right' caption='([[3LOG]]) is a 4 chain structure of MbtI with sequence from [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3LOG OCA].'> | ||
| - | |||
| - | |||
==Introduction== | ==Introduction== | ||
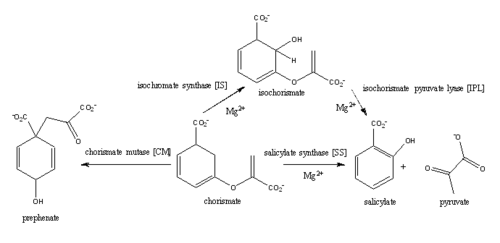
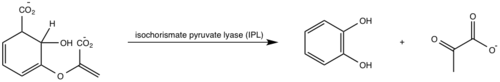
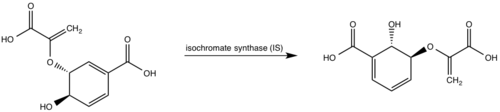
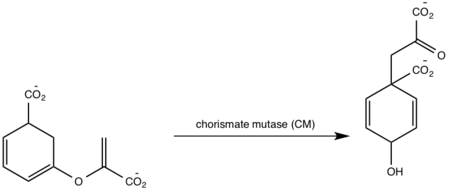
| - | <scene name='69/694235/3log/12'>Salicylate synthase</scene> from [http://en.wikipedia.org/wiki/''Mycobacterium_tuberculosis''] (MtbI) is a highly promiscuous enzyme that has four distinct activities ''in vivo'': [http://en.wikipedia.org/wiki/Isochorismate_synthase isochorismate synthase] (IS), [http://www.proteopedia.org/wiki/index.php/Isochorismate_pyruvate_lyase isochorismate pyruvate lyase] (IPL), [http://www.rcsb.org/pdb/results/results.do?outformat=&qrid=8A8773E9&tabtoshow=Current salicylate synthase] (SS) and [http://en.wikipedia.org/wiki/Chorismate_mutase chorismate mutate] (CM)(Ferrer 2012). MtbI belongs to the chorismate- | + | <scene name='69/694235/3log/12'>Salicylate synthase</scene> from [http://en.wikipedia.org/wiki/''Mycobacterium_tuberculosis''] (MtbI) is a highly promiscuous enzyme that has four distinct activities ''in vivo'': [http://en.wikipedia.org/wiki/Isochorismate_synthase isochorismate synthase] (IS), [http://www.proteopedia.org/wiki/index.php/Isochorismate_pyruvate_lyase isochorismate pyruvate lyase] (IPL), [http://www.rcsb.org/pdb/results/results.do?outformat=&qrid=8A8773E9&tabtoshow=Current salicylate synthase] (SS) and [http://en.wikipedia.org/wiki/Chorismate_mutase chorismate mutate] (CM)(Ferrer 2012). MtbI belongs to the chorismate-utilizing enzyme family, which consists of structural homologues (<scene name='69/694235/Irp9/5'>Ipr9</scene>, <scene name='69/694235/Menf/3'>MenF</scene>, <scene name='69/694235/Entc/3'>EntC</scene>, and <scene name='69/694235/Mbti/3'>MbtI</scene>) that isomerize chromate to isochorismate and share a fold of two α/β subdomains, each comprising of a antiparallel β-sheet with helices packed against it(Ferrer 2012, Lamb 2011). These enzymes are present in bacteria, fungi, plants and apicomplexan parasites and catalyze the initial reactions of menaquinone, siderophore, and tryptophan biosynthesis(ferrer 2012, Lamb 2011, Voss 1999). The IS, IPL, and SS activity of MbtI require the presence of a magnesium ion within the active site, while CM activity is only observed in absence of the magnesium cation(ferrer 2012). IS, IPL, and SS activity are also modulated by the pH of the medium(ferrer 2012). Isochorismate is the primary product at pH values below 7.5 and salicylate is the primary product formed at pH 8(ferrer 2012, Zwahlen 2006). |
| - | The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating [http://en.wikipedia.org/wiki/Siderophore siderophore], mycobactin T, in ''Mycobacterium tuberculosis'' (Figure 1)<ref name= "5a">PMID:22607697</ref>. This complex secondary metabolite is essential for both virulence and survival of ''M. tuberculosis''(ferrer 2012, Voss 1999, Harrison 2006). Therefore, inhibitors of salicylate synthase may serve as potential TB therapies with a novel mode of action <ref name= "1a"> DOI: 10.1002/cmdc/201000137</ref> <ref name= "2a">PMID:23108268</ref> <ref name= "7a">Voss, James J., Kerry Rutter, Benjamin G. Schroedor, Hua Su, and YaQi Zhu. "The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages." Proceedings of the National Academy of Sciences 97.3 (2000): 1252-57. Web. 14 Mar. 2015.</ref> <ref name= "5a"/> <ref name= "4a">DOI:10.1021/bi2009739</ref> <ref name= "9a">PMID:14982443</ref> | + | |
| + | The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating [http://en.wikipedia.org/wiki/Siderophore siderophore], mycobactin T, in ''Mycobacterium tuberculosis'' (Figure 1)<ref name= "5a">PMID:22607697</ref>. Mycobactin T is synthesized by the proteins encoded by the ''mbt'' and ''mbt2'' gene clusters <ref name="5a"/>. The gene Rv2386c is essential for the in vitro growth of "M. tuberculosis" and codes the enzyme MbtI (turvey, 2010). This complex secondary metabolite is essential for both virulence and survival of ''M. tuberculosis''(ferrer 2012, Voss 1999, Harrison 2006). Therefore, inhibitors of salicylate synthase may serve as potential TB therapies with a novel mode of action <ref name= "1a"> DOI: 10.1002/cmdc/201000137</ref> <ref name= "2a">PMID:23108268</ref> <ref name= "7a">Voss, James J., Kerry Rutter, Benjamin G. Schroedor, Hua Su, and YaQi Zhu. "The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages." Proceedings of the National Academy of Sciences 97.3 (2000): 1252-57. Web. 14 Mar. 2015.</ref> <ref name= "5a"/> <ref name= "4a">DOI:10.1021/bi2009739</ref> <ref name= "9a">PMID:14982443</ref> | ||
[[Image:Pathways.png|500 px|center|thumb|'''Figure 1:''' Pathways catalyzed by wild-type MbtI<ref>PMID:22307014</ref>.]] | [[Image:Pathways.png|500 px|center|thumb|'''Figure 1:''' Pathways catalyzed by wild-type MbtI<ref>PMID:22307014</ref>.]] | ||
==Structure== | ==Structure== | ||
| + | |||
[[Image:Active_site_cleft.png|300 px|left|thumb|Figure 2: This shows a single sub unit of MbtI, with the active site cleft located at the lower left hand side of the image.]] | [[Image:Active_site_cleft.png|300 px|left|thumb|Figure 2: This shows a single sub unit of MbtI, with the active site cleft located at the lower left hand side of the image.]] | ||
| - | The crystal asymmetric unit was found to contain <scene name='69/694235/3log/11'> four MbtI molecules</scene>, however crystal packing and size exclusion chromatography data suggest a monomeric enzyme. There are no significant structural changes between the four monomers excepts from the localized differences in the active site <ref name= "3a">PMID 15342575</ref>. The overall molecular structure consist of a polypeptide of 450 residues that forms one large single domain with a similar fold to other chromate-utilizing enzymes <ref name="3a"/>. The core of the protein is formed by <scene name='69/694234/Beta_sheets/1'>21 Beta sheets </scene>folded into a twisted beta-sandwich. The protein's core is then surrounded by <scene name='69/694235/Beta_sheets/4'>10 alpha helices</scene><ref name="3a"/>. | + | |
| + | The crystal asymmetric unit was found to contain <scene name='69/694235/3log/11'> four MbtI molecules</scene>, however crystal packing and size exclusion chromatography data suggest a monomeric enzyme <ref name= "3a">PMID 15342575</ref>. There are no significant structural changes between the four monomers excepts from the localized differences in the active site <ref name= "3a">PMID 15342575</ref>. The overall molecular structure consist of a polypeptide of 450 residues that forms one large single domain with a similar fold to other chromate-utilizing enzymes <ref name="3a"/>. The core of the protein is formed by <scene name='69/694234/Beta_sheets/1'>21 Beta sheets </scene>folded into a twisted beta-sandwich. The protein's core is then surrounded by <scene name='69/694235/Beta_sheets/4'>10 alpha helices</scene><ref name="3a"/>. The active site was identified by comparison to the product bound forms of Irp9 and TrpE and is situated in a cleft that is about 12Å in length, 10Å deep, and 7Å wide. One side of the groove is formed by β21, C-terminal helix, and α11. The other side of the groove is formed by β16-17 loop, helix α7, and β15-α6 loop. The β19-20 and β12-13 loops make up the bottom of the active side cleft (Harrison 2006) | ||
| + | |||
<scene name='69/694235/Alpha_helics/2'>TextToBeDisplayed</scene> | <scene name='69/694235/Alpha_helics/2'>TextToBeDisplayed</scene> | ||
| Line 20: | Line 22: | ||
== Structural highlights == | == Structural highlights == | ||
| - | + | MbtI structure has a mobile element (residues 323 to 327) that can adopt a <scene name='69/694235/Irp9_closed_state/2'>closed</scene> or <scene name='69/694235/2g5f_with_open_loop/1'>open conformation</scene> depending on whether or not ligands are bound to the active site(Harrison 2006). The closed conformation partially obstructs the active site. <ref name= "5a"/>. | |
| - | Inhibition studies have also shown a switch in binding mode at the MbtI active site for inhibitors carrying a substituted enolpyruvyl group compared to the chorismate substrate. Crystal structures and fluorescent-based thermal shift assays show that substituents larger than a methyl group are accommodated in the active site of MbtI through localized flexibility in the peptide backbone. Positioning of the active site residues of MbtI with the inhibitor AMT is highly similar to the closed form of MbtI <ref name= "5a"/>. | + | Inhibition studies have also shown a switch in binding mode at the MbtI active site for inhibitors carrying a substituted enolpyruvyl group compared to the chorismate substrate(Chi 2006, Turvey 2012, Turvey 2010). Crystal structures and fluorescent-based thermal shift assays show that substituents larger than a methyl group are accommodated in the active site of MbtI through localized flexibility in the peptide backbone(Chi 2006). Positioning of the active site residues of MbtI in [[3ST6]] with the <scene name='69/694235/3st6_structure_bindingpocket/2'>inhibitor AMT bound</scene> is highly similar to the closed form of MbtI as see in <scene name='69/694235/3log_bindingpocket/1'>3log</scene><ref name= "5a"/>. |
==Molecular Mechanism== | ==Molecular Mechanism== | ||
Revision as of 17:33, 26 April 2015
Contents |
Mycobacterium tuberculosis salicylate synthase (Mbt1)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Chi G, Manos-Turvey A, O'Connor PD, Johnston JM, Evans GL, Baker EN, Payne RJ, Lott JS, Bulloch EM. Implications of Binding Mode and Active Site Flexibility for Inhibitor Potency against the Salicylate Synthase from Mycobacterium tuberculosis. Biochemistry. 2012 Jun 7. PMID:22607697 doi:10.1021/bi3002067
- ↑ 2.0 2.1 doi: https://dx.doi.org/10.1002/cmdc/201000137
- ↑ 3.0 3.1 Manos-Turvey A, Cergol KM, Salam NK, Bulloch EM, Chi G, Pang A, Britton WJ, West NP, Baker EN, Lott JS, Payne RJ. Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site. Org Biomol Chem. 2012 Dec 14;10(46):9223-36. doi: 10.1039/c2ob26736e. Epub 2012, Oct 29. PMID:23108268 doi:http://dx.doi.org/10.1039/c2ob26736e
- ↑ Voss, James J., Kerry Rutter, Benjamin G. Schroedor, Hua Su, and YaQi Zhu. "The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages." Proceedings of the National Academy of Sciences 97.3 (2000): 1252-57. Web. 14 Mar. 2015.
- ↑ Lamb AL. Pericyclic reactions catalyzed by chorismate-utilizing enzymes. Biochemistry. 2011 Sep 6;50(35):7476-83. doi: 10.1021/bi2009739. Epub 2011 Aug, 12. PMID:21823653 doi:http://dx.doi.org/10.1021/bi2009739
- ↑ He Z, Stigers Lavoie KD, Bartlett PA, Toney MD. Conservation of mechanism in three chorismate-utilizing enzymes. J Am Chem Soc. 2004 Mar 3;126(8):2378-85. PMID:14982443 doi:http://dx.doi.org/10.1021/ja0389927
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ 8.0 8.1 8.2 8.3 Nicoloff H, Arsene-Ploetze F, Malandain C, Kleerebezem M, Bringel F. Two arginine repressors regulate arginine biosynthesis in Lactobacillus plantarum. J Bacteriol. 2004 Sep;186(18):6059-69. PMID:15342575 doi:http://dx.doi.org/10.1128/JB.186.18.6059-6069.2004
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ He Z, Stigers Lavoie KD, Bartlett PA, Toney MD. Conservation of mechanism in three chorismate-utilizing enzymes. J Am Chem Soc. 2004 Mar 3;126(8):2378-85. PMID:14982443 doi:http://dx.doi.org/10.1021/ja0389927
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Tuberculosis (TB). Ed. Sam Posner. Centers for Disease Control and Prevention, n.d. Web. 9 Apr. 2015.
- ↑ De Voss, James J., Kerry Rutter, Benjamin G. Schroeder, Hua Su, and YaQi Zhu. The salicylate-derived mycobacterium siderophore of Mycobacterium tuberculosis are essential for growth in macrophages. "Proceedings of the National Science Academy" 97.3 (2000): 1252-57. Web. 5 Apr. 2015.
Student contributors
Stephanie Raynor and Robin Gagnon
Related pdb files and proteopedia pages
3D structures of isochorismate pyruvate lyase
3log – MtIPL/isochorismate synthase - Mycobacterium tuberculosis
3rv6, 3rv7, 3rv8, 3rv9, 3st6, 3veh - MtIPL/isochorismate synthase + inhibitor
2h9c – PaIPL residues 1-99 – Pseudomonas aeruginosa
2h9d - PaIPL + pyruvate
3LOG
3D structure of isochorismate synthase
2eua, 3bzm, 3bzn - MenF from E. coli
3os6 - DhbC from Bacillus anthracis
3gse - MenF from Yersinia pestis
3hwo - EntC
3D structure of salicylate synthase
3veh - MbtI with inhibitor methylAMT
3st6 - MbtI with isochorismate analogue inhibitor
3rv6 (Phenyl R-group), 3rv7 (Isopropyl R-group), 3rv8 (Cyclopropyl R-group), 3rv9 (Ethyl R-group) - MbtI with inhibitor
2fn0, 2fn1 (with products salicylate and pyruvate) - Irp9 from Yersinia enterocolitica
2i6y - MbtI