This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox 985
From Proteopedia
| Line 1: | Line 1: | ||
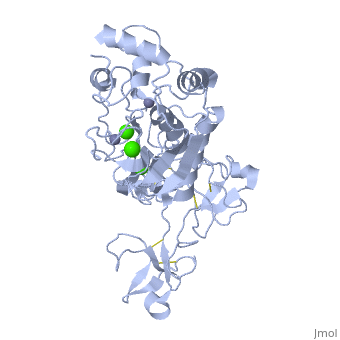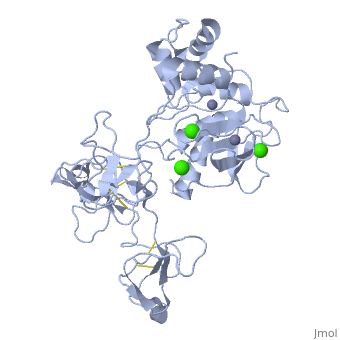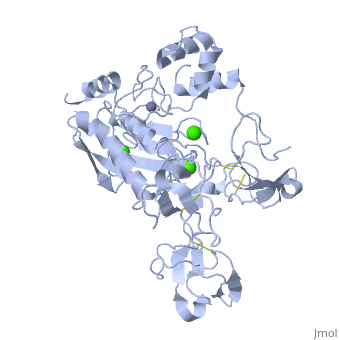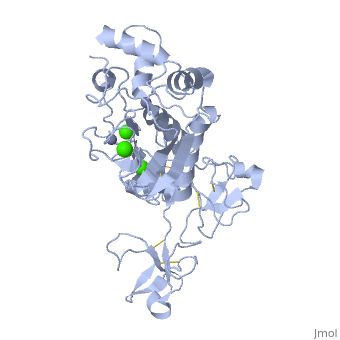
MMP family members share similar fundamental structural characteristics and are classified according to their substrate specificity. By this classification, MMP-9 belongs to the gelatinase subgroup and is known as gelatinase B due to its ability to degrade gelatin. MMP9 is composed of the following domains: a gelatin-binding domain consisting of three fibronectin type II units, a catalytic domain containing the zinc-binding site, a proline-rich type V collagen-homologous domain and a hemopexin-like domain. The zinc binding motif HEXXHXXGXXH in the catalytic domain, and the “cysteine switch” motif PRCGXPD in the propeptide are common structural signatures, where three histidines in the zinc binding motif coordinate and the cysteine in the propetide coordinate with the catalytic zinc ion. MMP9 is produced by the several cell types, normal alveolar macrophages and granulocytes. | MMP family members share similar fundamental structural characteristics and are classified according to their substrate specificity. By this classification, MMP-9 belongs to the gelatinase subgroup and is known as gelatinase B due to its ability to degrade gelatin. MMP9 is composed of the following domains: a gelatin-binding domain consisting of three fibronectin type II units, a catalytic domain containing the zinc-binding site, a proline-rich type V collagen-homologous domain and a hemopexin-like domain. The zinc binding motif HEXXHXXGXXH in the catalytic domain, and the “cysteine switch” motif PRCGXPD in the propeptide are common structural signatures, where three histidines in the zinc binding motif coordinate and the cysteine in the propetide coordinate with the catalytic zinc ion. MMP9 is produced by the several cell types, normal alveolar macrophages and granulocytes. | ||
| - | <StructureSection load=' | + | <StructureSection load='1l6j' size='340' side='right' caption='Human matrix metalloproteinase MMP9 (gelatinase B),1l6j' scene=''> |
This is a default text for your page '''Matrix metalloproteinase 9'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | This is a default text for your page '''Matrix metalloproteinase 9'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
| Line 23: | Line 23: | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| - | < | + | <ref/> |
Revision as of 15:15, 4 May 2015
MMP family members share similar fundamental structural characteristics and are classified according to their substrate specificity. By this classification, MMP-9 belongs to the gelatinase subgroup and is known as gelatinase B due to its ability to degrade gelatin. MMP9 is composed of the following domains: a gelatin-binding domain consisting of three fibronectin type II units, a catalytic domain containing the zinc-binding site, a proline-rich type V collagen-homologous domain and a hemopexin-like domain. The zinc binding motif HEXXHXXGXXH in the catalytic domain, and the “cysteine switch” motif PRCGXPD in the propeptide are common structural signatures, where three histidines in the zinc binding motif coordinate and the cysteine in the propetide coordinate with the catalytic zinc ion. MMP9 is produced by the several cell types, normal alveolar macrophages and granulocytes.
| |||||||||||
References
Cite error: Invalid <ref> tag;
refs with no content must have a name