We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 977
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
Monoamine Oxidase A (MAOA) | Monoamine Oxidase A (MAOA) | ||
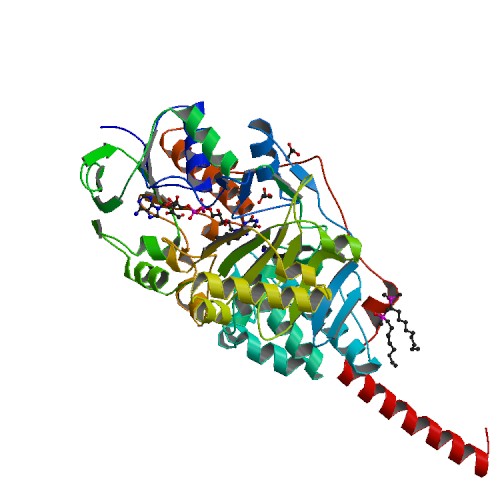
<StructureSection load='2z5x' size='340' side='right' caption='Structure of Monoamine Oxidase A (MAOA)' scene=''>[[Image:2z5x_bio_r_500.jpg]] | <StructureSection load='2z5x' size='340' side='right' caption='Structure of Monoamine Oxidase A (MAOA)' scene=''>[[Image:2z5x_bio_r_500.jpg]] | ||
| - | MAOAs are the most abundant isoform of Monoamine oxidases. The majority are located inside the noradrenergic neurons on the outer mitochondrial membranes. <ref>Machado-Vieira, R., & G. Mallinger, A. (2012). Abnormal Function of Monoamine Oxidase-A in comorbid Major Depressive disorder and Cardiovascular Disease: Pathophysical and Theraputic Implications (Review). Molecular Medicine Reports, 6, 915-922. Retrieved April 2, 2015, from Scifinder.</ref | + | MAOAs are the most abundant isoform of Monoamine oxidases. The majority are located inside the noradrenergic neurons on the outer mitochondrial membranes. <ref>Machado-Vieira, R., & G. Mallinger, A. (2012). Abnormal Function of Monoamine Oxidase-A in comorbid Major Depressive disorder and Cardiovascular Disease: Pathophysical and Theraputic Implications (Review). Molecular Medicine Reports, 6, 915-922. Retrieved April 2, 2015, from Scifinder.</ref> |
== Function == | == Function == | ||
| - | The major function of this enzyme is to degrade biogenic amines. MAOAs catalyze the oxidation of serotonin, norepinephrine, and dopamine. Then hydrolyze the oxidative products to give aldehydes or ketones. <ref>Gaweska, Helena. "Structures and Mechanism of the Monoamine Oxidase Family." BioMol Concepts 2 (2011): 365-77. Print.</ref> | + | The major function of this enzyme is to degrade biogenic amines. MAOAs catalyze the oxidation of serotonin, norepinephrine, and dopamine. Then hydrolyze the oxidative products to give aldehydes or ketones. <ref>Gaweska, Helena. "Structures and Mechanism of the Monoamine Oxidase Family." BioMol Concepts 2 (2011): 365-77. Print.</ref><scene name='70/700893/Catalytic_site/2'>The catalytic site of MAOA with out the highlighted active site residues.</scene> |
== Disease == | == Disease == | ||
MAOAs remove amino functional groups to leave an oxidized oxygen atom behind, and producing ammonia and hydrogen peroxide. The hydrogen peroxide is a major mediator in the production of hydroxyl radicals in the body. Free hydroxyl radicals in the body have detrimental effects on several organs especially the brain. MAOAs are associated with major depressive disorder and cardiovascular disease. Increased activity of this enzyme can lead to downstream dysregulations of elevation of oxidative stresses, increased platelet activity, and cytokine levels.<ref>Machado-Vieira, R., & G. Mallinger, A. (2012). Abnormal Function of Monoamine Oxidase-A in comorbid Major Depressive disorder and Cardiovascular Disease: Pathophysical and Theraputic Implications (Review). Molecular Medicine Reports, 6, 915-922. Retrieved April 2, 2015, from Scifinder.</ref> | MAOAs remove amino functional groups to leave an oxidized oxygen atom behind, and producing ammonia and hydrogen peroxide. The hydrogen peroxide is a major mediator in the production of hydroxyl radicals in the body. Free hydroxyl radicals in the body have detrimental effects on several organs especially the brain. MAOAs are associated with major depressive disorder and cardiovascular disease. Increased activity of this enzyme can lead to downstream dysregulations of elevation of oxidative stresses, increased platelet activity, and cytokine levels.<ref>Machado-Vieira, R., & G. Mallinger, A. (2012). Abnormal Function of Monoamine Oxidase-A in comorbid Major Depressive disorder and Cardiovascular Disease: Pathophysical and Theraputic Implications (Review). Molecular Medicine Reports, 6, 915-922. Retrieved April 2, 2015, from Scifinder.</ref> | ||
Revision as of 15:31, 4 May 2015
Monoamine Oxidase A (MAOA)
| |||||||||||
References
- ↑ Machado-Vieira, R., & G. Mallinger, A. (2012). Abnormal Function of Monoamine Oxidase-A in comorbid Major Depressive disorder and Cardiovascular Disease: Pathophysical and Theraputic Implications (Review). Molecular Medicine Reports, 6, 915-922. Retrieved April 2, 2015, from Scifinder.
- ↑ Gaweska, Helena. "Structures and Mechanism of the Monoamine Oxidase Family." BioMol Concepts 2 (2011): 365-77. Print.
- ↑ Machado-Vieira, R., & G. Mallinger, A. (2012). Abnormal Function of Monoamine Oxidase-A in comorbid Major Depressive disorder and Cardiovascular Disease: Pathophysical and Theraputic Implications (Review). Molecular Medicine Reports, 6, 915-922. Retrieved April 2, 2015, from Scifinder.
- ↑ Machado-Vieira, R., & G. Mallinger, A. (2012). Abnormal Function of Monoamine Oxidase-A in comorbid Major Depressive disorder and Cardiovascular Disease: Pathophysical and Theraputic Implications (Review). Molecular Medicine Reports, 6, 915-922. Retrieved April 2, 2015, from Scifinder.
- ↑ Gaweska, Helena. "Structures and Mechanism of the Monoamine Oxidase Family." BioMol Concepts 2 (2011): 365-77. Print.
- ↑ E. Jones, T., Giurato, L., Guccione, S., & R. Ramsay, R. (2007). Interactions of Imidazoline Ligands with the Active Site of Purified Monoamine Oxidase A. The FEBS Journal, 1567-1575. Retrieved January 1, 2015, from Scifinder.