Sandbox Reserved 1051
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | = | + | =Trehalose-''O''-mycolyltransferase Ag85C= |
==Introduction== | ==Introduction== | ||
Antigen 85C is one of three homologous protein components of the Ag85 complex in the cell wall of ''M. tuberculosis''. This serine esterase enzyme catalyzes the transfer of mycolyl groups, characteristic components of the cell wall of mycobacteria. Several three dimensional structures of Ag85C have been solved, including the wild type enzyme as well as active site variants due to site-directed mutagenesis and covalent modification. | Antigen 85C is one of three homologous protein components of the Ag85 complex in the cell wall of ''M. tuberculosis''. This serine esterase enzyme catalyzes the transfer of mycolyl groups, characteristic components of the cell wall of mycobacteria. Several three dimensional structures of Ag85C have been solved, including the wild type enzyme as well as active site variants due to site-directed mutagenesis and covalent modification. | ||
| Line 10: | Line 10: | ||
The unusually thick and waxy cell wall of [http://en.wikipedia.org/wiki/Mycobacterium mycobacteria] is primarily composed of [http://en.wikipedia.org/wiki/Peptidoglycan peptidoglycans], [http://en.wikipedia.org/wiki/Arabinogalactan arbinogalactans], and [http://en.wikipedia.org/wiki/Mycolic_acid mycolic acids]. The mycolic acids, which have very long carbon chains and exhibit extreme hydrophobicity, are responsible for forming the outermost layer of the cell wall, thus creating a hydrophobic envelope surrounding the mycobacterium. Much of the mycolic acid content of the cell wall is in the form of esters (trehalose-6-monomycolate, TMM) and bis-esters (trehalose-6,6'-dimycolate, TDM, cord factor) of trehalose. | The unusually thick and waxy cell wall of [http://en.wikipedia.org/wiki/Mycobacterium mycobacteria] is primarily composed of [http://en.wikipedia.org/wiki/Peptidoglycan peptidoglycans], [http://en.wikipedia.org/wiki/Arabinogalactan arbinogalactans], and [http://en.wikipedia.org/wiki/Mycolic_acid mycolic acids]. The mycolic acids, which have very long carbon chains and exhibit extreme hydrophobicity, are responsible for forming the outermost layer of the cell wall, thus creating a hydrophobic envelope surrounding the mycobacterium. Much of the mycolic acid content of the cell wall is in the form of esters (trehalose-6-monomycolate, TMM) and bis-esters (trehalose-6,6'-dimycolate, TDM, cord factor) of trehalose. | ||
| - | The antigen 85 (Ag85) complex in [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis ''Mycobacterium tuberculosis''] is composed of three homologous proteins: Ag85A, Ag85B, and Ag85C, encoded by the ''fbpA'', ''fbpB'', and ''fbpC'' genes, respectively. Each of these Ag85 components (A, B, and C) catalyze mycolyl transfer from one molecule of TMM to another (Figure 1) using a catalytic serine triad. | + | The antigen 85 (Ag85) complex in [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis ''Mycobacterium tuberculosis''] is composed of three homologous proteins: Ag85A, Ag85B, and Ag85C, encoded by the ''fbpA'', ''fbpB'', and ''fbpC'' genes, respectively. Each of these Ag85 components (A, B, and C) is an acyltransferase enzyme (EC 2.3.1.122) that can catalyze mycolyl transfer from one molecule of TMM to another (Figure 1) using a catalytic serine triad. |
NEED REFERENCES! | NEED REFERENCES! | ||
INSERT '''SIMPLE''' REACTION SCHEME AS FIGURE 1 (see Gobec et al.) | INSERT '''SIMPLE''' REACTION SCHEME AS FIGURE 1 (see Gobec et al.) | ||
===Clinical Relevance=== | ===Clinical Relevance=== | ||
| - | The hydrophobic envelope created by these mycolic acids creates a barrier against small hydrophilic molecules, such as Tuberculosis antibiotics. By targeting this mycoloyltransferase activity, inhibition of Ag85C offers potential for cell wall disruption and subsequent antibiotic targeting for normally drug-resistant '' | + | The hydrophobic envelope created by these mycolic acids creates a barrier against small hydrophilic molecules, such as Tuberculosis antibiotics. By targeting this mycoloyltransferase activity, inhibition of Ag85C offers potential for cell wall disruption and subsequent antibiotic targeting for normally drug-resistant ''Mycobacteria tuberculosis''. <ref>PMID: 10200974</ref> |
THIS SECTION STILL NEEDS A LOT OF WORK | THIS SECTION STILL NEEDS A LOT OF WORK | ||
==Structure== | ==Structure== | ||
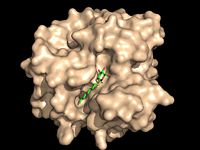
| - | [[Image:Substrate Binding 2D Surface.jpg|200 px|left|thumb|'''Figure | + | [[Image:Substrate Binding 2D Surface.jpg|200 px|left|thumb|'''Figure 2:''' Octylthioglucoside, a substrate analog, shown in the binding pocket of [http://www.rcsb.org/pdb/explore/explore.do?structureId=1VA5 Ag85C]]] |
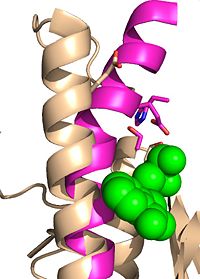
| - | + | Wild type Ag85C, in the absence of any specific ligands, was originally crystallized as a <scene name='69/694219/Dimer/1'>homodimer</scene>.<ref name="Ronning2000">PMID: 10655617</ref> The <scene name='69/694220/Secondary_structures/2'>secondary structure</scene> (shown in the monomeric form) is 38% α-helical (pink) and 16% β-sheet (yellow). The confrontation of a central β-sheet bordered by α–helices comprises an [http://en.wikipedia.org/wiki/Alpha/beta_hydrolase_fold α/β hydrolase fold] in Ag85C, a supersecondary structure that is highly conserved across serine hydrolases. The active site of Ag85C is composed of two adjoined binding pockets; there is carbohydrate binding pocket for the trehalose substrate, and there is a fatty acid binding pocket for the mycolic acid. As a result, trehalose monomycolate can effectively bind to the Ag85C active site. This binding pocket is shown in Figure 2, in which Ag85C is shown with a bound substrate mimic, octylthioglucoside (Figure 3). | |
| - | + | ||
== Enzymatic Activity == | == Enzymatic Activity == | ||
Revision as of 02:38, 13 May 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Trehalose-O-mycolyltransferase Ag85C
Introduction
Antigen 85C is one of three homologous protein components of the Ag85 complex in the cell wall of M. tuberculosis. This serine esterase enzyme catalyzes the transfer of mycolyl groups, characteristic components of the cell wall of mycobacteria. Several three dimensional structures of Ag85C have been solved, including the wild type enzyme as well as active site variants due to site-directed mutagenesis and covalent modification.
| |||||||||||
References
- ↑ Jackson M, Raynaud C, Laneelle MA, Guilhot C, Laurent-Winter C, Ensergueix D, Gicquel B, Daffe M. Inactivation of the antigen 85C gene profoundly affects the mycolate content and alters the permeability of the Mycobacterium tuberculosis cell envelope. Mol Microbiol. 1999 Mar;31(5):1573-87. PMID:10200974
- ↑ Ronning DR, Klabunde T, Besra GS, Vissa VD, Belisle JT, Sacchettini JC. Crystal structure of the secreted form of antigen 85C reveals potential targets for mycobacterial drugs and vaccines. Nat Struct Biol. 2000 Feb;7(2):141-6. PMID:10655617 doi:10.1038/72413