Regulator of G protein signaling
From Proteopedia
(Difference between revisions)
| Line 31: | Line 31: | ||
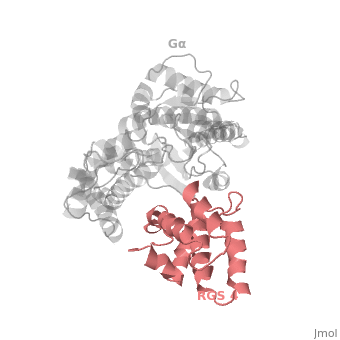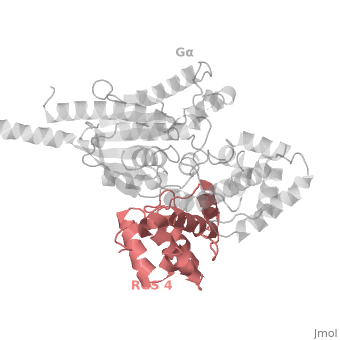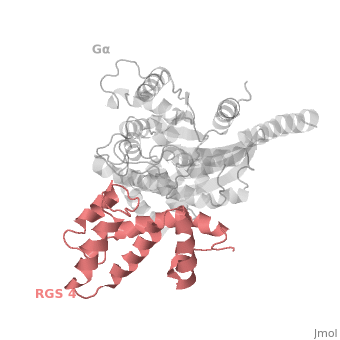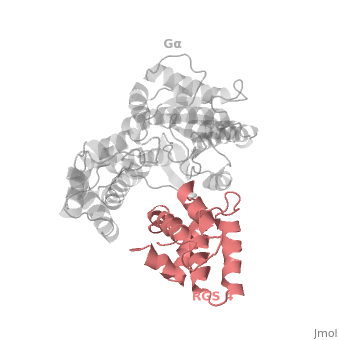
<scene name='70/701447/Rgs_monomer/13'>Monomer structure of RGS4</scene> in green cartoon diagram: The RGS4 domain corresponds to an array of nine α-helices that fold into two small subdomains. The terminal subdomain contains the N and C termini of the box and is formed by α1, α2, α3, α8, and α9. Helices α1 and α9 lie in antiparallel orientation, juxtaposing the N and C termini of the box. The larger bundle subdomain, formed by α4, α5, α6, and α7, is a classic right-handed, antiparallel four-helix bundle. Both subdomains are required for GAP activity. | <scene name='70/701447/Rgs_monomer/13'>Monomer structure of RGS4</scene> in green cartoon diagram: The RGS4 domain corresponds to an array of nine α-helices that fold into two small subdomains. The terminal subdomain contains the N and C termini of the box and is formed by α1, α2, α3, α8, and α9. Helices α1 and α9 lie in antiparallel orientation, juxtaposing the N and C termini of the box. The larger bundle subdomain, formed by α4, α5, α6, and α7, is a classic right-handed, antiparallel four-helix bundle. Both subdomains are required for GAP activity. | ||
| - | Gα<sub>i1</sub> subunits adopt a conserved fold composed of <scene name='70/701447/All-helical-domain/ | + | Gα<sub>i1</sub> subunits adopt a conserved fold composed of <scene name='70/701447/All-helical-domain/6'>α helical domain</scene> (deepsky blue cartoon), a helical domain of six α helices and <scene name='70/701447/Gtpase_domain/2'>a GTPase domain</scene> (cornflower blue cartoon).The GTPase domain hydrolyzes GTP and provides most of Gα's binding surfaces for Gβγ, receptors, effectors and RGS proteins. The GTPase domain contains three flexible regions designated <scene name='70/701447/Gi-s1/6'>switch-I</scene> presented as blue sticks, <scene name='70/701447/Gi-s2/3'>switch-II</scene> presented as magenta sticks and <scene name='70/701447/Gi-s3/2'>switch-III</scene> presented as green sticks that change conformation in response to GTP binding and hydrolysis. The three switch regions of Gα<sub>i1</sub>: residues 176–184, 201–215, and 233–241, respectively . <ref>PMID: 9108480</ref> |
</StructureSection> | </StructureSection> | ||
Revision as of 08:55, 17 May 2015
Regulator of G protein signaling (RGS) interactions with G proteins – RGS4-Gαi as a model structure.
| |||||||||||
References
- ↑ Kosloff M, Travis AM, Bosch DE, Siderovski DP, Arshavsky VY. Integrating energy calculations with functional assays to decipher the specificity of G protein-RGS protein interactions. Nat Struct Mol Biol. 2011 Jun 19;18(7):846-53. doi: 10.1038/nsmb.2068. PMID:21685921 doi:http://dx.doi.org/10.1038/nsmb.2068
- ↑ Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br J Pharmacol. 2006 Jan;147 Suppl 1:S46-55. PMID:16402120 doi:http://dx.doi.org/10.1038/sj.bjp.0706405
- ↑ Tesmer JJ, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4--activated G(i alpha1): stabilization of the transition state for GTP hydrolysis. Cell. 1997 Apr 18;89(2):251-61. PMID:9108480
Proteopedia Page Contributors and Editors (what is this?)
Ali Asli, Denise Salem, Michal Harel, Joel L. Sussman, Jaime Prilusky