User:Rana Saad/The human GABAb receptor
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
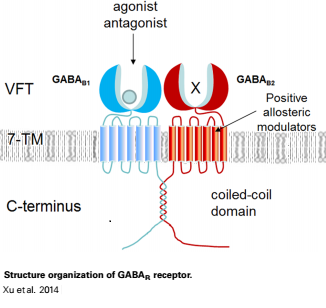
The mammalian '''GABA<sub>B</sub>''' receptor is a class C [http://proteopedia.org/wiki/index.php/G_protein-coupled_receptor#3D_Structures_of_G_protein-coupled_receptors G-protein coupled receptor]<ref>PMID:23237917</ref>. Its structure is similar to the [[Metabotropic glutamate receptor|metabotropic glutamate receptors]] (mGluR) ligand binding domain. The GABA<sub>B</sub> receptor is central to inhibitory neurotransmission in the brain and so is considered a good candidate for treatments against alcoholism, stress and a number of brain diseases<ref>PMID:19913201</ref>. | The mammalian '''GABA<sub>B</sub>''' receptor is a class C [http://proteopedia.org/wiki/index.php/G_protein-coupled_receptor#3D_Structures_of_G_protein-coupled_receptors G-protein coupled receptor]<ref>PMID:23237917</ref>. Its structure is similar to the [[Metabotropic glutamate receptor|metabotropic glutamate receptors]] (mGluR) ligand binding domain. The GABA<sub>B</sub> receptor is central to inhibitory neurotransmission in the brain and so is considered a good candidate for treatments against alcoholism, stress and a number of brain diseases<ref>PMID:19913201</ref>. | ||
| - | The GABA<sub>B</sub> receptor causes the opening of the K<sup>+</sup> channels in the postsynaptic membrane, bringing the neuron closer to the [https://www.youtube.com/watch?v=4kx9_0YwShE equilibrium potential] of K<sup>+</sup>, producing [http://en.wikipedia.org/wiki/Hyperpolarization_(biology) hyperpolarization]. As a result the Ca<sup>+2</sup> channels in the presynaptic terminal close and neurotransmitter release stops. In addition GABA<sub>B</sub> receptor function lead to reduce the activity of adenylyl cyclase and decrease the cell’s conductance to Ca<sup>+2</sup>.[http://physrev.physiology.org/content/84/3/835.short]. | + | The GABA<sub>B</sub> receptor causes the opening of the K<sup>+</sup> channels in the postsynaptic membrane, bringing the neuron closer to the [https://www.youtube.com/watch?v=4kx9_0YwShE equilibrium potential] of K<sup>+</sup>, producing [http://en.wikipedia.org/wiki/Hyperpolarization_(biology) hyperpolarization]. As a result, the Ca<sup>+2</sup> channels in the presynaptic terminal close, and neurotransmitter release stops. In addition GABA<sub>B</sub> receptor function lead to reduce the activity of adenylyl cyclase and decrease the cell’s conductance to Ca<sup>+2</sup>.[http://physrev.physiology.org/content/84/3/835.short]. |
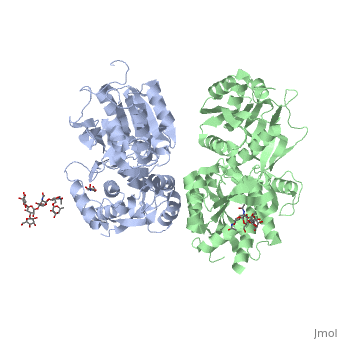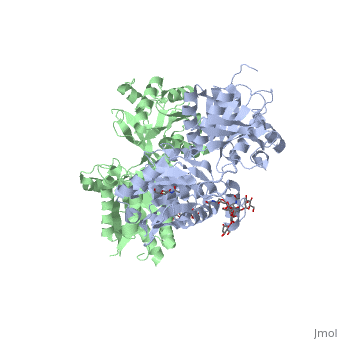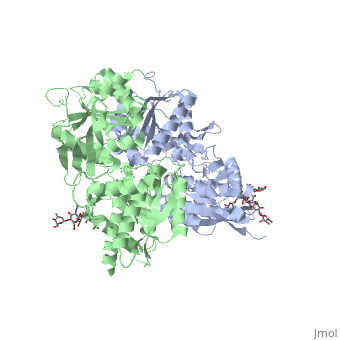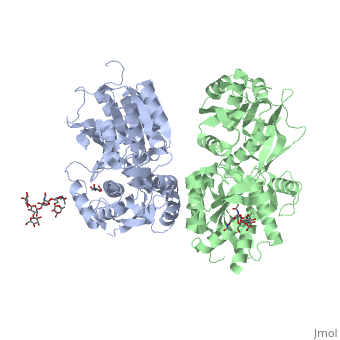
[[Image:GABAb.receptor.cartoon2.png|thumb|250px]] | [[Image:GABAb.receptor.cartoon2.png|thumb|250px]] | ||
=='''''Structure'''''== | =='''''Structure'''''== | ||
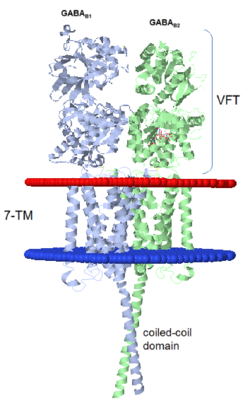
| - | GABA<sub>B</sub> receptor functions as an obligatory heterodimer subunit of '''GABA<sub>B1</sub>''' (GBR1) and '''GABA<sub>B2</sub>''' (GBR2). '''GBR1''' (blue) is responsible for ligand-binding (chain A). '''GBR2''' (green), on the other hand, is responsible for G protein coupling | + | GABA<sub>B</sub> receptor functions as an obligatory heterodimer subunit of '''GABA<sub>B1</sub>''' (GBR1) and '''GABA<sub>B2</sub>''' (GBR2). '''GBR1''' (blue) is responsible for ligand-binding (chain A). '''GBR2''' (green), on the other hand, is responsible for the G protein coupling (chain B). The GABA<sub>B</sub> receptor is one of only a few obligate receptor heterodimer currently known. There is no crystal or NMR structure of the complete receptor since it have extracellular and inter cellular regions. |
| - | + | The formation of a functional GABA<sub>B</sub> receptor requires the heterodimeric assembly of GBR1 and GBR2 receptor subunits. Both consist of a seven-helix transmembrane domain composed of a large an N-terminal extracellular domain, venus flytrap (VFT), it is called like this because it is like the [http://en.wikipedia.org/wiki/Venus_flytrap venus flytrap] while binding agonist and antagonist, and a C-terminal intracellular domain which is responsible for GABA<sub>B</sub> receptor heterodimerization. | |
==='''The extracellular domain VFT'''=== | ==='''The extracellular domain VFT'''=== | ||
The VFT contains two lobe-shaped domains: LB1 and LB2, which are connected by three short loops. | The VFT contains two lobe-shaped domains: LB1 and LB2, which are connected by three short loops. | ||
LB1 and LB2 are <scene name='70/701448/Gbr2_subunit/1'>αβ-folds composed of central β sheet flanked by an α helix</scene><ref>PMID:24305054</ref>. | LB1 and LB2 are <scene name='70/701448/Gbr2_subunit/1'>αβ-folds composed of central β sheet flanked by an α helix</scene><ref>PMID:24305054</ref>. | ||
| - | ====''''' | + | ===='''''The agonist and antagonist binding'''''==== |
All of the [http://en.wikipedia.org/wiki/Agonist agonists] and [http://en.wikipedia.org/wiki/Receptor_antagonist antagonists] bind the '''extracellular VFT module situated at the crevice between the LB1 and LB2 domains of the GBR1 subunit'''. | All of the [http://en.wikipedia.org/wiki/Agonist agonists] and [http://en.wikipedia.org/wiki/Receptor_antagonist antagonists] bind the '''extracellular VFT module situated at the crevice between the LB1 and LB2 domains of the GBR1 subunit'''. | ||
Revision as of 17:02, 9 July 2015
| |||||||||||