Regulator of G protein signaling
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
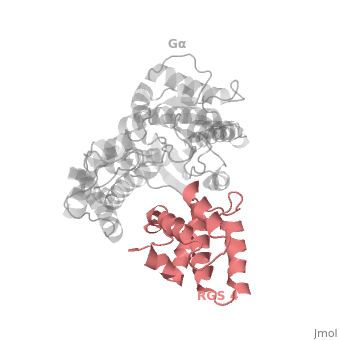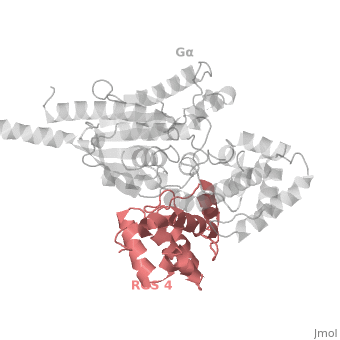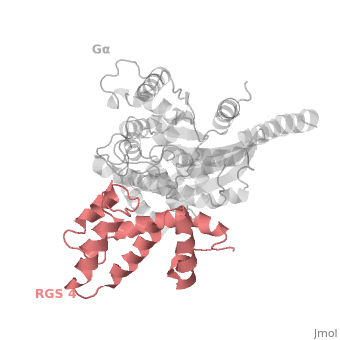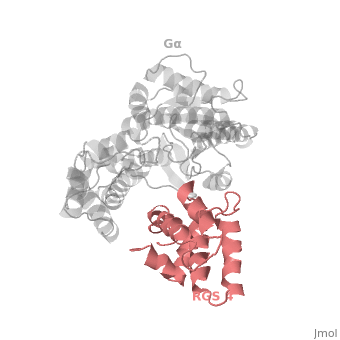
RGS proteins are selective for binding to the transition state of Gα(GTP → GDP + P<sub>i</sub>). | RGS proteins are selective for binding to the transition state of Gα(GTP → GDP + P<sub>i</sub>). | ||
| - | Like many signaling proteins, RGS proteins comprise a large and diverse family. In human genome, Thirty-seven RGS proteins are encoded by gene loci; this collection of related proteins has been divided into 10 different subfamilies based on the relatedness of their RGS domain sequence and their multiple domain architectures. The major subfamily contains About 20 ‘canonical’ RGS proteins that can in theory downregulate any of the 16 activated Gα subunits, although in practice they interact only with members of the G<sub>i</sub> and G<sub>q</sub> families. In these proteins, the ~120-residue RGS homology domain functions as a GTPase-activating protein (GAP) for GTP-bound Gα subunits. | + | Like many signaling proteins, RGS proteins comprise a large and diverse family. In human genome, Thirty-seven RGS proteins are encoded by gene loci; this collection of related proteins has been divided into 10 different subfamilies based on the relatedness of their RGS domain sequence and their multiple domain architectures. The major subfamily contains About 20 ‘canonical’ RGS proteins that can in theory downregulate any of the 16 activated Gα subunits, although in practice they interact only with members of the G<sub>i</sub> and G<sub>q</sub> families. In these proteins, the ~120-residue RGS homology domain functions as a GTPase-activating protein (GAP) for GTP-bound Gα subunits. In addition to these domains, diverse proteins subfamilies include additional protein-protein interaction domains beyond their signature RGS domain with Gα GAP activity. R7-subfamily members share a multi-domain protein architecture composed of DEP and GGL domains on the N-terminal side of the RGS domain. R12-subfamily members possess a tandem repeat of Ras binding domains (RBDs) and a single GoLoco motif. |
<imagemap> | <imagemap> | ||
| Line 25: | Line 25: | ||
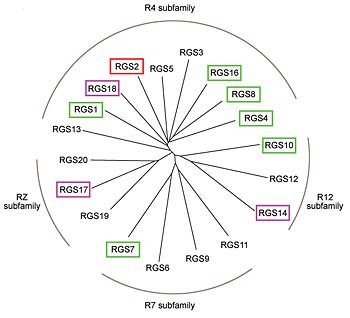
RGS proteins whose activity was tested are colored by their GAP activity, RGS proteins with high GAP activity (green), RGS proteins with low but discernible activities (purple) and RGS2 had no measurable activity (red). | RGS proteins whose activity was tested are colored by their GAP activity, RGS proteins with high GAP activity (green), RGS proteins with low but discernible activities (purple) and RGS2 had no measurable activity (red). | ||
<ref>PMID: 21685921</ref> | <ref>PMID: 21685921</ref> | ||
| - | |||
| - | In addition to these domains, diverse proteins subfamilies include additional protein-protein interaction domains beyond their signature RGS domain with Gα GAP activity. R7-subfamily members share a multi-domain protein architecture composed of DEP and GGL domains on the N-terminal side of the RGS domain. R12-subfamily members possess a tandem repeat of Ras binding domains (RBDs) and a single GoLoco motif. | ||
== Structural highlights == | == Structural highlights == | ||
Revision as of 13:44, 10 July 2015
Regulator of G protein signaling (RGS) interactions with G proteins – RGS4-Gαi as a model structure.
| |||||||||||
References
- ↑ Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br J Pharmacol. 2006 Jan;147 Suppl 1:S46-55. PMID:16402120 doi:http://dx.doi.org/10.1038/sj.bjp.0706405
- ↑ Kosloff M, Travis AM, Bosch DE, Siderovski DP, Arshavsky VY. Integrating energy calculations with functional assays to decipher the specificity of G protein-RGS protein interactions. Nat Struct Mol Biol. 2011 Jun 19;18(7):846-53. doi: 10.1038/nsmb.2068. PMID:21685921 doi:http://dx.doi.org/10.1038/nsmb.2068
- ↑ Tesmer JJ, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4--activated G(i alpha1): stabilization of the transition state for GTP hydrolysis. Cell. 1997 Apr 18;89(2):251-61. PMID:9108480
- ↑ Kosloff M, Travis AM, Bosch DE, Siderovski DP, Arshavsky VY. Integrating energy calculations with functional assays to decipher the specificity of G protein-RGS protein interactions. Nat Struct Mol Biol. 2011 Jun 19;18(7):846-53. doi: 10.1038/nsmb.2068. PMID:21685921 doi:http://dx.doi.org/10.1038/nsmb.2068
Proteopedia Page Contributors and Editors (what is this?)
Ali Asli, Denise Salem, Michal Harel, Joel L. Sussman, Jaime Prilusky