FMDV 3C
From Proteopedia
| Line 9: | Line 9: | ||
[[Image:FMDV_3C_Prot.jpeg]] | [[Image:FMDV_3C_Prot.jpeg]] | ||
== Function == | == Function == | ||
| - | + | ||
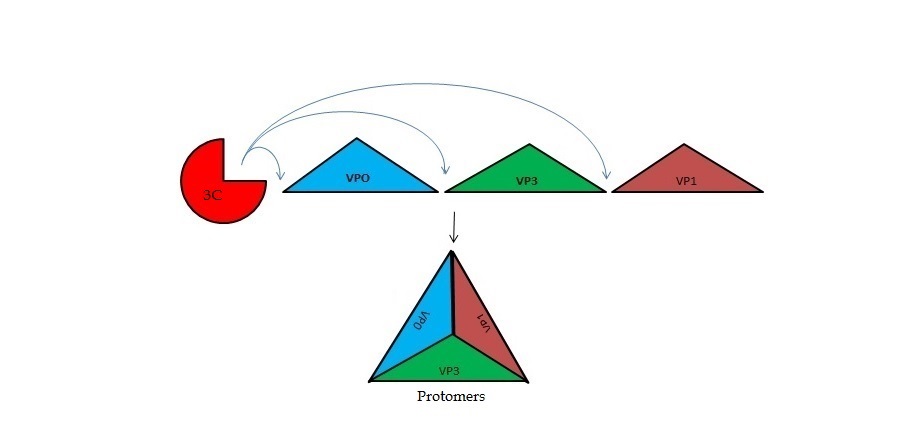
| + | The 3C only known function of the viral 3C protease was processing of the viral polyprotein, until recent research discovered interactions with host cell proteins. The 3C protease has been shown to induce proteolytic cleavage of Histone H3. As well as rapid inhibition of host cell protein synthesis by cleavage of translation initiation factors eIF4A, eIF4G between residues E143 and V144. | ||
| + | 3C is also able to induce Golgi Fragmentation, causing fragmentation of early, medial, and late Golgi compartments with the most pronounced effect being on early Golgi compartments. Research has found that the Golgi fragments were still able to receive membrane proteins from the endoplasmic reticulum however they were unable to transfer protein to the plasma membrane. | ||
| + | |||
| + | 3C has been shown to be able to cleave the C terminus of the nuclear RNA binding protein SAM 68 resulting in redistribution to the cytoplasm. This was particularly interesting because of the fact that Sam68 has been shown to effect FMDV post entry events by interacting with the internal ribosomal entry site within the 5′ non-translated region of the FMDV genome, and Sam68 knockdown decreased FMDV IRES-driven activity in vitro suggesting that it could modulate translation of the viral genome. | ||
| + | |||
Revision as of 12:58, 31 July 2015
FMDV 3C Protease
| |||||||||||
References
Klump, W., O. Marquardt, and P.H. Hofschneider, Biologically active protease of foot and mouth disease virus is expressed from cloned viral cDNA in Escherichia coli. Proc Natl Acad Sci U S A, 1984. 81(11): p. 3351-5.
Grubman, M.J., M. Zellner, and J. Wagner, Antigenic comparison of the polypeptides of foot-and-mouth disease virus serotypes and other picornaviruses. Virology, 1987. 158(1): p. 133-40.
Vakharia, V.N., et al., Proteolytic processing of foot-and-mouth disease virus polyproteins expressed in a cell-free system from clone-derived transcripts. J Virol, 1987. 61(10): p. 3199-207.
Grubman, M.J. and B. Baxt, Translation of foot-and-mouth disease virion RNA and processing of the primary cleavage products in a rabbit reticulocyte lysate. Virology, 1982. 116(1): p. 19-30.
5.ablanian, G.M. and M.J. Grubman, Characterization of the foot-and-mouth disease virus 3C protease expressed in Escherichia coli. Virology, 1993. 197(1): p. 320-7.
Ryan, M.D., G.J. Belsham, and A.M. King, Specificity of enzyme-substrate interactions in foot-and-mouth disease virus polyprotein processing. Virology, 1989. 173(1): p. 35-45.
Clarke, B.E. and D.V. Sangar, Processing and assembly of foot-and-mouth disease virus proteins using subgenomic RNA. J Gen Virol, 1988. 69 ( Pt 9): p. 2313-25.
Grubman, M.J., et al., Identification of the active-site residues of the 3C proteinase of foot-and-mouth disease virus. Virology, 1995. 213(2): p. 581-9.
Falk, M.M., et al., Foot-and-mouth disease virus protease 3C induces specific proteolytic cleavage of host cell histone H3. J Virol, 1990. 64(2): p. 748-56.
Belsham, G.J., G.M. McInerney, and N. Ross-Smith, Foot-and-mouth disease virus 3C protease induces cleavage of translation initiation factors eIF4A and eIF4G within infected cells. J Virol, 2000. 74(1): p. 272-80.
Strong, R. and G.J. Belsham, Sequential modification of translation initiation factor eIF4GI by two different foot-and-mouth disease virus proteases within infected baby hamster kidney cells: identification of the 3Cpro cleavage site. J Gen Virol, 2004. 85(Pt 10): p. 2953-62.
Devaney, M.A., et al., Leader protein of foot-and-mouth disease virus is required for cleavage of the p220 component of the cap-binding protein complex. J Virol, 1988. 62(11): p. 4407-9.
Medina, M., et al., The two species of the foot-and-mouth disease virus leader protein, expressed individually, exhibit the same activities. Virology, 1993. 194(1): p. 355-9.
Li, W., et al., Cleavage of translation initiation factor 4AI (eIF4AI) but not eIF4AII by foot-and-mouth disease virus 3C protease: identification of the eIF4AI cleavage site. FEBS Lett, 2001. 507(1): p. 1-5.
Zhou, Z., et al., Foot-and-mouth disease virus 3C protease induces fragmentation of the Golgi compartment and blocks intra-Golgi transport. J Virol, 2013. 87(21): p. 11721-9.
Armer, H., et al., Foot-and-mouth disease virus, but not bovine enterovirus, targets the host cell cytoskeleton via the nonstructural protein 3Cpro. J Virol, 2008. 82(21): p. 10556-66.
Lawrence, P., E.A. Schafer, and E. Rieder, The nuclear protein Sam68 is cleaved by the FMDV 3C protease redistributing Sam68 to the cytoplasm during FMDV infection of host cells. Virology, 2012. 425(1): p. 40-52.
Wang, D., et al., Foot-and-mouth disease virus 3C protease cleaves NEMO to impair innate immune signaling. J Virol, 2012. 86(17): p. 9311-22.
Martinez-Salas, E. and E. Domingo, Effect of expression of the aphthovirus protease 3C on viral infection and gene expression. Virology, 1995. 212(1): p. 111-20.
Birtley, J.R., et al., Crystal structure of foot-and-mouth disease virus 3C protease. New insights into catalytic mechanism and cleavage specificity. J Biol Chem, 2005. 280(12): p. 11520-7.
Sweeney, T.R., et al., Structural and mutagenic analysis of foot-and-mouth disease virus 3C protease reveals the role of the beta-ribbon in proteolysis. J Virol, 2007. 81(1): p. 115-24.
references [1]