Regulator of G protein signaling
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
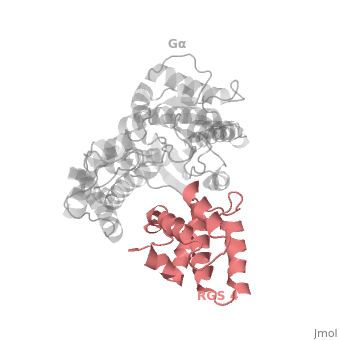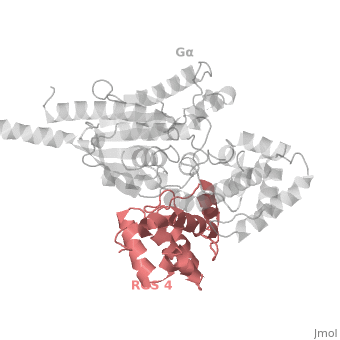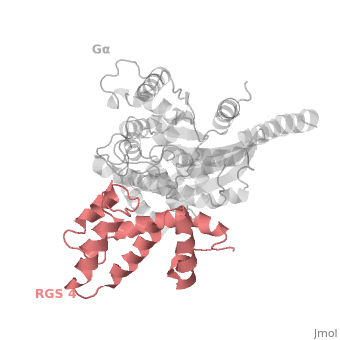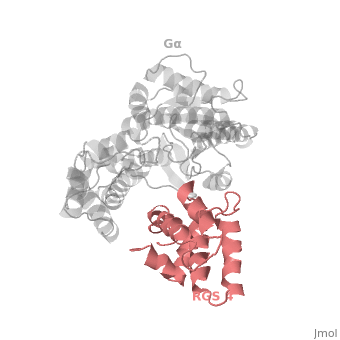
==Regulator of G protein signaling (RGS) interactions with G proteins – RGS4-Gα<sub>i</sub> as a model structure.== | ==Regulator of G protein signaling (RGS) interactions with G proteins – RGS4-Gα<sub>i</sub> as a model structure.== | ||
| - | <StructureSection load='1agr' size='340' side='right' caption=' | + | <StructureSection load='1agr' size='340' side='right' caption='Rat regulator of G-protein signalling 4 (grey, yellow) complex with guanine nucleotide-binding protein α subunit (magenta, red) (PDB code [[1agr]])' scene='70/701447/Gi-rgs4/9' pspeed='8'> |
== Heterotrimeric G-proteins family == | == Heterotrimeric G-proteins family == | ||
Human heterotrimeric G-proteins are derived from 35 genes: 16 encoding α subunits, 5 β and 14 γ subunits. | Human heterotrimeric G-proteins are derived from 35 genes: 16 encoding α subunits, 5 β and 14 γ subunits. | ||
| Line 13: | Line 13: | ||
== RGS proteins == | == RGS proteins == | ||
| - | Regulator of G-proteins signaling (RGS) proteins play a critical role in many G protein-dependent signaling pathways. Thus, RGS proteins have been implicated in a wide range of pathologies, including cancer, hypertension, arrhythmias, drug abuse and schizophrenia. RGS proteins ‘turn off’ heterotrimeric (αβγ) G-proteins and thereby determine the duration of G protein–mediated signaling events. Therefore, RGS proteins function as GTPase Activating Proteins (GAPs), and this GAP activity is mediated by allosteric interactions. | + | '''Regulator of G-proteins signaling''' (RGS) proteins play a critical role in many G protein-dependent signaling pathways. Thus, RGS proteins have been implicated in a wide range of pathologies, including cancer, hypertension, arrhythmias, drug abuse and schizophrenia. RGS proteins ‘turn off’ heterotrimeric (αβγ) G-proteins and thereby determine the duration of G protein–mediated signaling events. Therefore, RGS proteins function as GTPase Activating Proteins (GAPs), and this GAP activity is mediated by allosteric interactions. |
RGS proteins are selective for binding to the transition state of Gα(GTP → GDP + P<sub>i</sub>). | RGS proteins are selective for binding to the transition state of Gα(GTP → GDP + P<sub>i</sub>). | ||
| Line 38: | Line 38: | ||
On the other side, Gα subunits participate in a range of interactions with a variety of other proteins. Therefore, they have interfaces that interact selectively with receptors, effector subfamilies and RGS proteins. However, <scene name='70/701447/Gi-rgs4_interface/4'>Gα residues</scene> from Gα<sub>i</sub> subfamily members that interact specifically with RGS proteins are highly conserved (red spheres). These Gα Residues located on Gα switch regions interact with Significant & Conserved RGS residues because of the pivotal role of the switch regions in GTP hydrolysis that is catalyzed by RGS proteins. On the other hand, Gα residues located in switch regions II and III and multiple residues in the Gα all-helical domain interact with Modulatory RGS residues.<ref name="Mickey2011" /> For example, one important conserved RGS4 residue that projects into the active site of Gα<sub>i</sub> is <scene name='70/701447/Gi-rgs4-asn128/2'>Asn-128</scene> shown as blue sticks, which contacts the side chains of three Gα residues: Lys-180, Gln-204, and Glu-207 shown as green, magenta, and red sticks respectively.<ref name="Tesmer97" /> A unique modulatory RGS4 residue that interacts with Gα<sub>i</sub> is <scene name='70/701447/Gi-rgs4_arg166/6'>Arg-166</scene> shown as blue sticks which forms salt bridge with the positively charged side chain of Glu-116 shown as magenta sticks located in the helical domain of Gα subunit. | On the other side, Gα subunits participate in a range of interactions with a variety of other proteins. Therefore, they have interfaces that interact selectively with receptors, effector subfamilies and RGS proteins. However, <scene name='70/701447/Gi-rgs4_interface/4'>Gα residues</scene> from Gα<sub>i</sub> subfamily members that interact specifically with RGS proteins are highly conserved (red spheres). These Gα Residues located on Gα switch regions interact with Significant & Conserved RGS residues because of the pivotal role of the switch regions in GTP hydrolysis that is catalyzed by RGS proteins. On the other hand, Gα residues located in switch regions II and III and multiple residues in the Gα all-helical domain interact with Modulatory RGS residues.<ref name="Mickey2011" /> For example, one important conserved RGS4 residue that projects into the active site of Gα<sub>i</sub> is <scene name='70/701447/Gi-rgs4-asn128/2'>Asn-128</scene> shown as blue sticks, which contacts the side chains of three Gα residues: Lys-180, Gln-204, and Glu-207 shown as green, magenta, and red sticks respectively.<ref name="Tesmer97" /> A unique modulatory RGS4 residue that interacts with Gα<sub>i</sub> is <scene name='70/701447/Gi-rgs4_arg166/6'>Arg-166</scene> shown as blue sticks which forms salt bridge with the positively charged side chain of Glu-116 shown as magenta sticks located in the helical domain of Gα subunit. | ||
</StructureSection> | </StructureSection> | ||
| + | ==3D structures of regulator of G-protein signalling== | ||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| + | |||
| + | |||
| + | * Regulator of G-protein signalling 1 | ||
| + | |||
| + | **[[2bv1]] – hRGS1 – human<br /> | ||
| + | |||
| + | * Regulator of G-protein signalling 3 | ||
| + | |||
| + | **[[2f5y]] – hRGS3 PDZ domain<br /> | ||
| + | |||
| + | * Regulator of G-protein signalling 4 | ||
| + | |||
| + | **[[1agr]] – RGS4 + guanine nucleotide-binding protein subunit α – rat <br /> | ||
| + | |||
| + | * Regulator of G-protein signalling 6 | ||
| + | |||
| + | **[[2es0]] – hRGS6<br /> | ||
| + | |||
| + | * Regulator of G-protein signalling 7 | ||
| + | |||
| + | **[[2a72]] – hRGS7<br /> | ||
| + | **[[2d5j]] – hRGS7 - NMR<br /> | ||
| + | |||
| + | * Regulator of G-protein signalling 10 | ||
| + | |||
| + | **[[2ihb]] – hRGS10 + guanine nucleotide-binding protein subunit α <br /> | ||
| + | |||
| + | * Regulator of G-protein signalling 14 | ||
| + | |||
| + | **[[2om2]] – hRGS14 GOLOCO motif + guanine nucleotide-binding protein subunit α <br /> | ||
| + | }} | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 11:03, 6 January 2016
Regulator of G protein signaling (RGS) interactions with G proteins – RGS4-Gαi as a model structure.
| |||||||||||
3D structures of regulator of G-protein signalling
Updated on 06-January-2016
References
- ↑ Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br J Pharmacol. 2006 Jan;147 Suppl 1:S46-55. PMID:16402120 doi:http://dx.doi.org/10.1038/sj.bjp.0706405
- ↑ 2.0 2.1 2.2 Kosloff M, Travis AM, Bosch DE, Siderovski DP, Arshavsky VY. Integrating energy calculations with functional assays to decipher the specificity of G protein-RGS protein interactions. Nat Struct Mol Biol. 2011 Jun 19;18(7):846-53. doi: 10.1038/nsmb.2068. PMID:21685921 doi:http://dx.doi.org/10.1038/nsmb.2068
- ↑ 3.0 3.1 Tesmer JJ, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4--activated G(i alpha1): stabilization of the transition state for GTP hydrolysis. Cell. 1997 Apr 18;89(2):251-61. PMID:9108480
Proteopedia Page Contributors and Editors (what is this?)
Ali Asli, Denise Salem, Michal Harel, Joel L. Sussman, Jaime Prilusky