Function
Inosine monophosphate dehydrogenase (IMPDH) is part of the GTP biosynthesis and catalyzes the conversion of inosine monophosphate to xanthosine monophosphate while reducing NAD. IMPDH is inhibited by ribavirin and mycophenolic acid[1].
Disease
IMPDH level of activity is suggested to determine whether acute leukemia cells proliferate or differentiate[2].
Structural highlights
An Insight to the Dynamics of Conserved Water Mediated Salt Bridge Interaction and Inter-Domain Recognition in hIMPDH Isoforms [3]
Dynamic personality of protein is inherent in Nature and necessary for performing the chemical reaction in living cells. The functions of proteins / enzymes and their physical properties are also governed by their dynamic characters which requires addition of a fourth dimension, time and atomic resolution. Detail investigation of dynamic and X-ray structures of protein or their complexes with different molecules can unfold the structure-function relationship of protein. Presumably, the dynamic propensity of protein is not only determine the chemical transformations/ reaction mechanism of biosynthetic processes but it also vividly illustrated the insight of topological feature of inhibitor. It is interesting in enzymology that Nature also installs some similar proteins which are almost have identical function, catalytic machinery, 3D-structures and are known as isoform. The salt bridge interaction (acidic and basic residues) as well as their conjugation through amphoteric water molecules (Acid---Water---Base) may be indispensable component of protein recognition, domain assemble and structure stabilization. The differential inter domain recognition through conserved water mediated salt bridge interaction in type -I and II isoforms (of human IMPDH) may open a new avenue towards their inhibitor development.
Inosine monophosphate dehydrogenase (IMPDH), the enzyme involves in the biosynthesis of GMP from IMP. hIMPDH has two isoforms, type –I and II that have almost similar sequence and functional motif. Increased level of isoform-II is found in CML (Chronic myelogenous leukemia), AML (Acute myelogenous leukemia), CLL (Chronic lymphocytic leukemia) and CML – BC (Blast crisis) blood cancer patients, whereas type-1 has house-keeping role in normal cells. So, the hIMPDH-II is led to new interest as an excellent target for design of isoform specific antileukemic agent. Developing the isoform selective inhibitor of hIMPDH-II seems difficult and challenging in structural biology because both isoforms (type-1 and II) have almost 84% sequence similarity, structural and functional identity and both are catalytically active. So, investigation of new ligand binding zone in hIMPDH could be very impressive and also be a novel research work for designing the isoform-specific inhibitor that could not interrupt the GMP biosynthesis rather it may act as negative allosteric modulator and indirectly control the excess XMP synthesis within the cell.
The structures of hIMPDH contain catalytic (394 residues) and a pair (60 residues length) of cystathionine-beta-synthase domains: CBS-1 (residues 112-171; colored in lime) and CBS-2 (residues 172-232; colored in green)) which are observed at periphery of protein or outside the catalytic barrel. But the sequence of CBS domains have subdivided the sequences of catalytic domains into IN (residues 28-111; colored in yellow) and IC (residues 233-504; colored in darkmagenta) sub-domains (see also image below). Structural segments of flap region (residues 400-450; colored in magenta) and CBS domains are almost inaccessible in X-ray structures of hIMPDH and nhIMPDH-II enzyme.
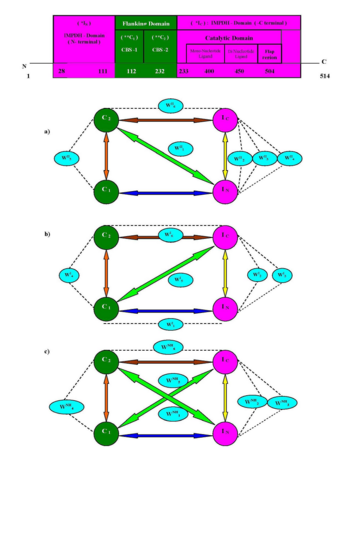
The schematic presentation of domain architecture in IMPDH–II enzyme.
Dynamics of conserved water mediated salt bridge in hIMPDH simulated structures
The six conserved hydrophilic sites which are stabilized the salt bridges (evolved during molecular simulation) in IMPDH seem very interesting and it shade some new light for the development of forthcoming science on IMPDH enzyme. The hydrophilic centers WII2, WII3 and WII4 of hIMPDH-II, WI2 and WI3 of hIMPDH-I, and WNH2, WNH3 of nhIMPDH-II structure are responsible for intra domain (IN --- IC) association, domain recognition through water mediated salt bridge. During dynamics, WII5, WI4 and WNH4 of , and (the water molecules are represented as red spheres) are solely involved in (C1 -- C2) intra domain recognition and gear the conformational orientation of flanking CBS domains. Another important structural role of WII1 and WNH1 are to stabilize the isoform-specific inter-domain (IN --- C2) in type –II IMPDH ( and ) structures or to connect the two terminal domains via water mediated salt bridge. Possibly the sequence dissimilarity (~ 15 % between type 1 and II) and structural topology of enzyme may control the dynamic behavior of water molecules in inter-domain clefts. Moreover, this inherent flexibility of water molecules in the hIMPDH-II simulated structure may influence the biochemical activities in CML cancer cells. The work put forward some light on the binding propensity of CBS domain with catalytic domains (IN and IC) via interaction of conserved water mediated salt bridges (WII1 and WII6 in , WI1, WI5 and WI6 in , WNH1, WNH2, WNH3, WNH5 and WNH6 in ) and their exclusive participation in recognition process.
During dynamics charges over the oxygen atom of water molecules are varied in the Lys --- Asp / Lys --- Glu salt bridges. Some charges on Lys (-Nz) are transferred to acidic oxygen of Asp / Glu residues and vise versa. However, in water mediated Arg --- Asp interaction, some charges are observed to flow from the basic nitrogen center to acidic oxygen atoms and the charges over water oxygen is also varied. During dynamics, the flow of charges through water molecules in Lys to Asp /Glu residues are observed to be bidirectional, whereas in case of Arg to Asp, it is unidirectional.
Topology of inhibitor for hIMPDH-II isoforms: Attempt to design of CML cancer drug
Stereospecific interaction or recognition of IN to C2 domain through are observed to be unique in hIMPDH–II, which is not observed in type I isoform (1JCN). The geometrical and electronic consequences of and their stereo chemical features (specially in CBS --- IN inter-domain recognition site) may be used to design the actual topology of inhibitor for hIMPDH-II isoform using water mimic inhibitor design protocol. Possibly, heterocyclic ligand with flexible structure containing two or three basic and hydrophilic groups with suitable spacer length may be implemented to design the isoform selective inhibitor for CML cancer.

