We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
PLC beta 3 Gq
From Proteopedia
(Difference between revisions)
| Line 26: | Line 26: | ||
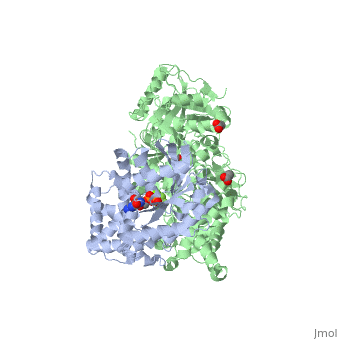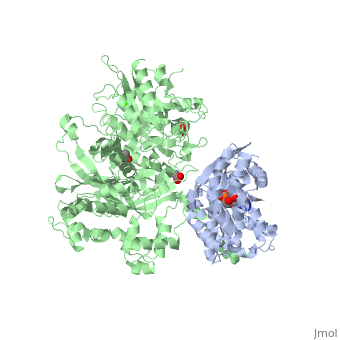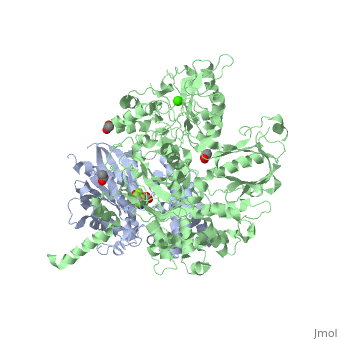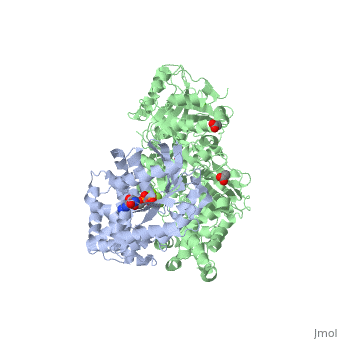
The canonical Gα effector-binding region of Gαq, located between α3 and switch 2, is occupied by a helix-turn-helix (Hα1/Hα2) that immediately follows the C2 domain of PLC-β3. <scene name='70/701452/Pro862/4'>Pro862</scene> of PLC-β3 lies within the turn between Hα1 and Hα2, makes extensive contacts with multiple residues of Gαq, and forms the center of a Gαq-binding interface. | The canonical Gα effector-binding region of Gαq, located between α3 and switch 2, is occupied by a helix-turn-helix (Hα1/Hα2) that immediately follows the C2 domain of PLC-β3. <scene name='70/701452/Pro862/4'>Pro862</scene> of PLC-β3 lies within the turn between Hα1 and Hα2, makes extensive contacts with multiple residues of Gαq, and forms the center of a Gαq-binding interface. | ||
| - | |||
<scene name='70/701452/Asn260/2'>Asn260</scene> is located at the active site of Gαq as part of a tight turn of PLC-β3 that is stabilized by Glu261 and underpinned by an extensive series of hydrogen bonds principally mediated by Asp256, Arg255 and Arg258. These residues are highly conserved in all PLC-βs, as are Asn251 and Leu267, which appear crucial in stabilizing the ends of the loop. | <scene name='70/701452/Asn260/2'>Asn260</scene> is located at the active site of Gαq as part of a tight turn of PLC-β3 that is stabilized by Glu261 and underpinned by an extensive series of hydrogen bonds principally mediated by Asp256, Arg255 and Arg258. These residues are highly conserved in all PLC-βs, as are Asn251 and Leu267, which appear crucial in stabilizing the ends of the loop. | ||
Revision as of 12:29, 17 August 2015
Unique bidirectional interactions of Phospholipase C beta 3 with G alpha Q
| |||||||||||
References
- ↑ Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, Wang X, Montell C, Kozasa T, Sondek J, Harden TK. Kinetic Scaffolding Mediated by a Phospholipase C-{beta} and Gq Signaling Complex. Science. 2010 Nov 12;330(6006):974-80. Epub 2010 Oct 21. PMID:20966218 doi:10.1126/science.1193438
- ↑ Lyon AM, Tesmer JJ. Structural insights into phospholipase C-beta function. Mol Pharmacol. 2013 Oct;84(4):488-500. doi: 10.1124/mol.113.087403. Epub 2013 Jul, 23. PMID:23880553 doi:http://dx.doi.org/10.1124/mol.113.087403
- ↑ Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, Wang X, Montell C, Kozasa T, Sondek J, Harden TK. Kinetic Scaffolding Mediated by a Phospholipase C-{beta} and Gq Signaling Complex. Science. 2010 Nov 12;330(6006):974-80. Epub 2010 Oct 21. PMID:20966218 doi:10.1126/science.1193438