Serum Paraoxonase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
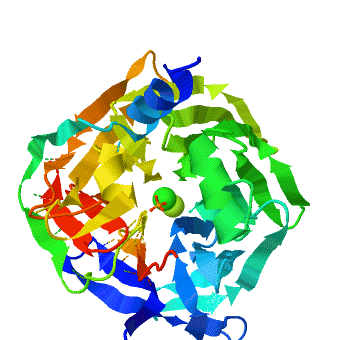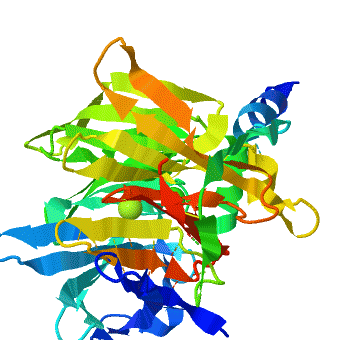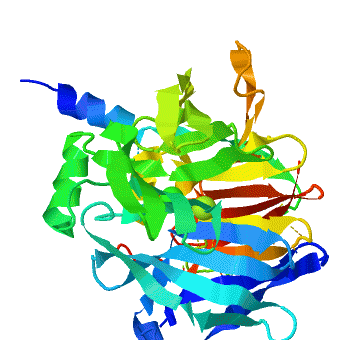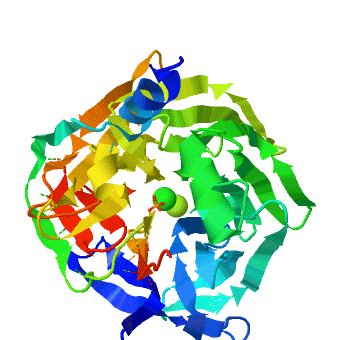
<StructureSection load='1v04' size='450' side='right' caption='PON1 - looking down 6-bladed propellers, Ca+2 (green) and phosphate (stick model) ions seen [[1v04]]' scene='1v04/Starting_scene/3'> | <StructureSection load='1v04' size='450' side='right' caption='PON1 - looking down 6-bladed propellers, Ca+2 (green) and phosphate (stick model) ions seen [[1v04]]' scene='1v04/Starting_scene/3'> | ||
| + | __NOTOC__ | ||
==Overview== | ==Overview== | ||
'''Serum paraoxonases''' (PONs) are a group of enzymes that play a key role in organophosphate detoxification and in prevention of atherosclerosis. There are three members in this family, PON1, PON2 and PON3, which share 60-70% nucleic acid identity. The most studied enzymes are the two isoenzymes of PON1, which differ in the residue at position 192 (Q/R). The primary activity of PON1 is lactone hydrolysis; however, this enzyme has many other activities. One of the interesting activities is the hydrolysis of organophosphates. PON1 can catalyze a variety of nerve agents such as cyclosarin, soman, etc. Therefore, it is aimed to be a nerve agent scavenger. In addition, PON1 has a role in prevention of atherosclerosis and is found to be attached to the high-density lipoprotein (HDL, “good cholesterol”). | '''Serum paraoxonases''' (PONs) are a group of enzymes that play a key role in organophosphate detoxification and in prevention of atherosclerosis. There are three members in this family, PON1, PON2 and PON3, which share 60-70% nucleic acid identity. The most studied enzymes are the two isoenzymes of PON1, which differ in the residue at position 192 (Q/R). The primary activity of PON1 is lactone hydrolysis; however, this enzyme has many other activities. One of the interesting activities is the hydrolysis of organophosphates. PON1 can catalyze a variety of nerve agents such as cyclosarin, soman, etc. Therefore, it is aimed to be a nerve agent scavenger. In addition, PON1 has a role in prevention of atherosclerosis and is found to be attached to the high-density lipoprotein (HDL, “good cholesterol”). | ||
| Line 17: | Line 18: | ||
The PON1 enzyme has theoretical biological importance as well as application for treatment of neurotoxins. Questions of the origins of enzyme promiscuity or the evolution of protein diversity may be illuminated by PON1's accidental low-level phosphotriesterase activity, and by the unintuitive effect of switching one amino acid in PON1 whereby it changes from a lactonase to a phosphotriesterase. Practically, a potent neurotoxin, "Paraoxon", and therefore a biochemical warfare threat, can be neutralized by phosphotriesterases. | The PON1 enzyme has theoretical biological importance as well as application for treatment of neurotoxins. Questions of the origins of enzyme promiscuity or the evolution of protein diversity may be illuminated by PON1's accidental low-level phosphotriesterase activity, and by the unintuitive effect of switching one amino acid in PON1 whereby it changes from a lactonase to a phosphotriesterase. Practically, a potent neurotoxin, "Paraoxon", and therefore a biochemical warfare threat, can be neutralized by phosphotriesterases. | ||
| - | |||
| - | See also [[Serum_Paraoxonase]] for a general review of issues relating to PON1 and the overall class of Serum Paraoxonases. A initial structural tour begins there as well. | ||
We experimentally solved two critical new PON1 structures. Previously solved in <scene name='Journal:JMB:2/Scene_1_2/3'>non-physiological conditions of pH 4.5</scene>, we have solved PON1 in <scene name='Journal:JMB:2/Scene_2_2/2'>physiological conditions of pH 6.5</scene>. While <scene name='Journal:JMB:2/Scene_3_2/1'>generally similar</scene>, as expected, there are some key differences. The side-chain of V346 within the active site pocket is <scene name='Journal:JMB:2/Scene_4_2/1'>rotated relative to the pH 4.5 structure</scene>, and the side-chains of F347 and H348 in the active site's 'second shell' <scene name='Journal:JMB:2/Scene_5_2/1'>adopted completely different rotamers</scene>. | We experimentally solved two critical new PON1 structures. Previously solved in <scene name='Journal:JMB:2/Scene_1_2/3'>non-physiological conditions of pH 4.5</scene>, we have solved PON1 in <scene name='Journal:JMB:2/Scene_2_2/2'>physiological conditions of pH 6.5</scene>. While <scene name='Journal:JMB:2/Scene_3_2/1'>generally similar</scene>, as expected, there are some key differences. The side-chain of V346 within the active site pocket is <scene name='Journal:JMB:2/Scene_4_2/1'>rotated relative to the pH 4.5 structure</scene>, and the side-chains of F347 and H348 in the active site's 'second shell' <scene name='Journal:JMB:2/Scene_5_2/1'>adopted completely different rotamers</scene>. | ||
Revision as of 08:28, 24 August 2015
| |||||||||||
3D structures of paraoxonase
1v04 – sPON + Ca + phosphate – synthetic
2vc7 – SsPON + decanoylamino-hydroxy-dehydro-thiophenium + Co + Fe – Sulfolobus solfataricus
2vc5 – SsPON + Co + Fe
4hho, 4hhq – sPON + Ca + Br
3sre – sPON + Ca + Br + phosphate
3srg – sPON + Ca + Br + oxoquinoline
References
- ↑ Harel M, Aharoni A, Gaidukov L, Brumshtein B, Khersonsky O, Meged R, Dvir H, Ravelli RB, McCarthy A, Toker L, Silman I, Sussman JL, Tawfik DS. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol. 2004 May;11(5):412-9. Epub 2004 Apr 18. PMID:15098021 doi:10.1038/nsmb767
- ↑ Gupta RD, Goldsmith M, Ashani Y, Simo Y, Mullokandov G, Bar H, Ben-David M, Leader H, Margalit R, Silman I, Sussman JL, Tawfik DS. Directed evolution of hydrolases for prevention of G-type nerve agent intoxication. Nat Chem Biol. 2011 Feb;7(2):120-5. Epub 2011 Jan 9. PMID:21217689 doi:10.1038/nchembio.510
- ↑ Ben-David M, Wieczorek G, Elias M, Silman I, Sussman JL, Tawfik DS. Catalytic metal ion rearrangements underline promiscuity and evolvability of a metalloenzyme. J Mol Biol. 2013 Mar 25;425(6):1028-38. doi: 10.1016/j.jmb.2013.01.009. Epub 2013, Jan 11. PMID:23318950 doi:10.1016/j.jmb.2013.01.009
- ↑ Ben-David M, Elias M, Filippi JJ, Dunach E, Silman I, Sussman JL, Tawfik DS. Catalytic Versatility and Backups in Enzyme Active Sites: The Case of Serum Paraoxonase 1. J Mol Biol. 2012 Mar 1. PMID:22387469 doi:10.1016/j.jmb.2012.02.042
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky, Eran Hodis, Boris Brumshtein, Moshe Ben-David