This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
DNA-protein interactions
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
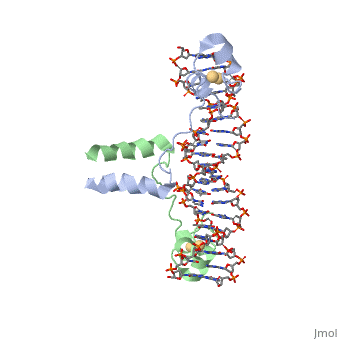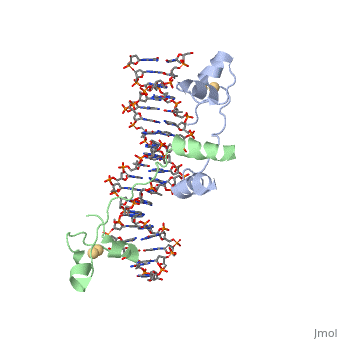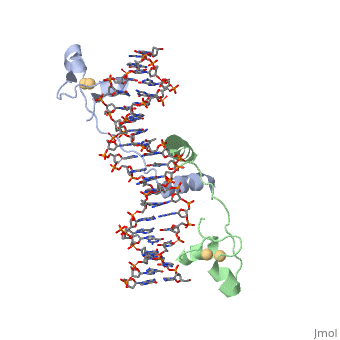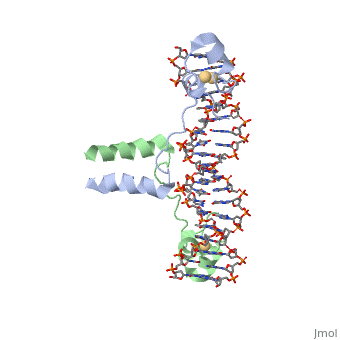
== Helix-Turn-Helix Interactions with DNA== | == Helix-Turn-Helix Interactions with DNA== | ||
| - | The first DNA binding domain characterized was the helix-turn-helix. In helix-turn-helix protein, two α helices are joined by a turn. In most cases | + | The first DNA binding domain characterized was the helix-turn-helix. In a <scene name='71/711660/Protein_rainbow/1'>helix-turn-helix protein</scene> such as the Cro repressor, two α helices are joined by a turn; there may be additional supporting structures, such as additional helices or beta strands, but this is the basic motif. In most cases, the <scene name='71/711660/C_terminal_helix/1'>C terminal helix</scene> contributes most to DNA recognition, and hence it is often called the "recognition helix". It binds to the major groove of DNA through a series of hydrogen bonds and various Van der Waals interactions with exposed bases. For example, Asn51 forms hydrogen bonds with both A219 and A220 of the DNA strand. The N-terminal alpha helix stabilizes the interaction between the interaction between protein and DNA, but does not play a particularly strong role in its recognition.[2] The recognition helix and its preceding helix always have the same relative orientation.[ |
== Leucine zippers == | == Leucine zippers == | ||
Revision as of 23:45, 2 September 2015
DNA-Protein interactions
| |||||||||||
References
This text shows how to insert references: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644