DNA-protein interactions
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== Zinc fingers == | == Zinc fingers == | ||
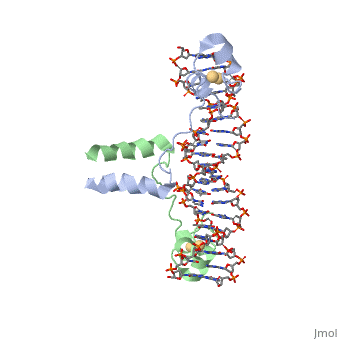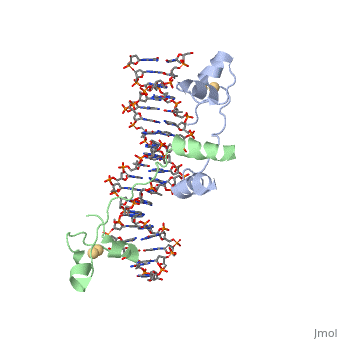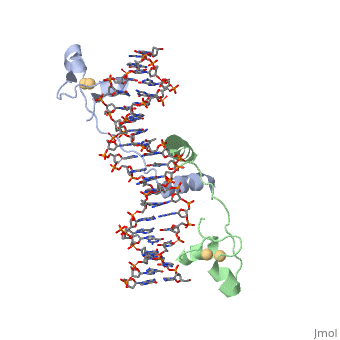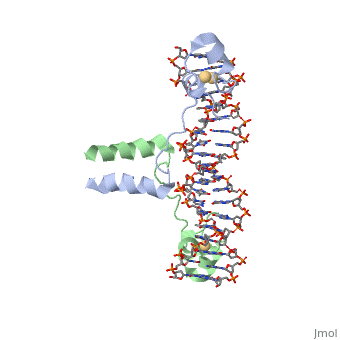
| - | <scene name='71/711660/Gal4/1'>GAL4</scene> is a transcription factor that induces genes required for the metabolism of galactose, specifically enzymes involved in the conversion of galactose to glucose, and is an example of a zinc finger DNA binding protein. The protein binds as a <scene name='Taylor_Gal4_Sandbox/Dimer/1'>dimer</scene> to a symmetrical 17-base-pair sequence. Each subunit folds into three distinct modules: a compact, <scene name='Taylor_Gal4_Sandbox/Metal_binding_domain/2'>metal binding domain</scene>(residues 8-40), an extended <scene name='Taylor_Gal4_Sandbox/Linker/1'>linker</scene>(41-49), and an <scene name='Taylor_Gal4_Sandbox/Dimerization/1'>alpha-helical dimerization element</scene> (50-64). The small, <scene name='Taylor_Gal4_Sandbox/Zn_binding/1'>Zn(2+)-containing domain</scene>, which contains two metal ions tetrahedrally coordinated by six cysteines. This metal binding domain recognizes a conserved CCG triplet at each end of the site through direct contacts with the <scene name='Taylor_Gal4_Sandbox/Major_groove/1'>major groove</scene>. A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4. | + | <scene name='71/711660/Gal4/1'>GAL4</scene> is a transcription factor that induces genes required for the metabolism of galactose, specifically enzymes involved in the conversion of galactose to glucose, and is an example of a zinc finger DNA binding protein. The protein binds as a <scene name='Taylor_Gal4_Sandbox/Dimer/1'>dimer</scene> to a symmetrical 17-base-pair sequence. Each subunit folds into three distinct modules: a compact, <scene name='Taylor_Gal4_Sandbox/Metal_binding_domain/2'>metal binding domain</scene>(residues 8-40), an extended <scene name='Taylor_Gal4_Sandbox/Linker/1'>linker</scene>(41-49), and an <scene name='Taylor_Gal4_Sandbox/Dimerization/1'>alpha-helical dimerization element</scene> (50-64). The small, <scene name='Taylor_Gal4_Sandbox/Zn_binding/1'>Zn(2+)-containing domain</scene>, which contains two metal ions tetrahedrally coordinated by six cysteines. This metal binding domain recognizes a conserved <scene name='71/711660/Ccg/1'>CCG triplet</scene> at each end of the site through direct contacts with the <scene name='Taylor_Gal4_Sandbox/Major_groove/1'>major groove</scene>, including hydrogen bonding between a <scene name='71/711660/Gal_4_lys_to_g/1'>lysine and the conserved G</scene>. A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4. |
Revision as of 03:22, 3 September 2015
DNA-Protein interactions
| |||||||||||
References
This text shows how to insert references: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644