DNA-protein interactions
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
== Leucine zippers == | == Leucine zippers == | ||
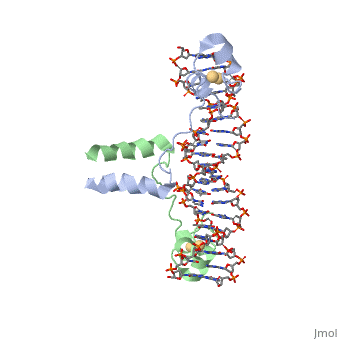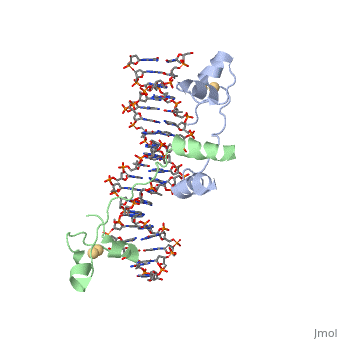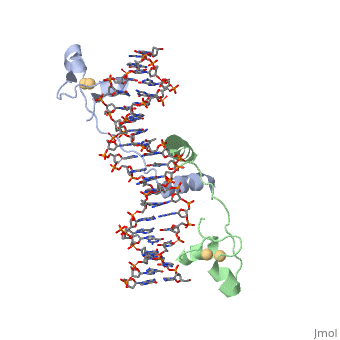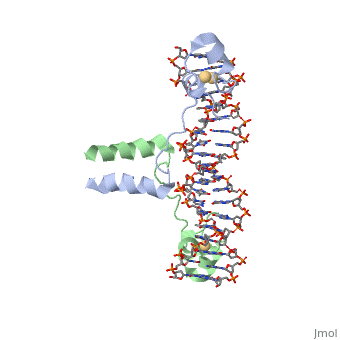
| - | The Basic Leucine Zipper Domain (<scene name='71/711660/Creb/1'>bZIP domain</scene>) is found in many DNA binding eukaryotic proteins, especially transcription factors such as the cAMP Responsive Element Binding (CREB) protein . One part of the domain contains a region that mediates sequence specific DNA binding properties via <scene name='71/711660/Creb_charge/2'>basic amino acids</scene> such as arginine and lysine. Since these basic amino acids would usually repel, the <scene name='71/711660/Creb_leu/1'>leucine zipper segment</scene> allows for dimerization of the protein. Notice the spacing of the leucine residues; they are spaced by 3, 4, or 7 amino acids, causing them to be on the same face of the alpha helix. this hydrophobic set of "zipper teeth" allows this region to interact with another monomer unit, pairing with the exact same residues to form a <scene name='71/711660/Creb_hydrophobic/1'>hydrophobic core</scene>, shown in grey. At the base of the leucine zipper, where the protein meets the DNA, is positioned a <scene name='71/711660/Creb__mg/1'>magnesium ion</scene>. While you would expect a divalent cation to be surrounded by negatively charged amino acids from the protein or backbone phosphates from the DNA, its inner chelation sphere is completely composed of <scene name='71/711660/Creb_h2o_mg/1'>water molecules</scene>. The next sphere of interactions include <scene name='71/711660/Creb_lys304/1'>two lysine residues</scene> (one from each protein chain) | + | The Basic Leucine Zipper Domain (<scene name='71/711660/Creb/1'>bZIP domain</scene>) is found in many DNA binding eukaryotic proteins, especially transcription factors such as the cAMP Responsive Element Binding (CREB) protein . One part of the domain contains a region that mediates sequence specific DNA binding properties via <scene name='71/711660/Creb_charge/2'>basic amino acids</scene> such as arginine and lysine. These basic residues can either interact ionically with the <scene name='71/711660/Creb_arg_p/1'>negatively charged backbone phosphate groups</scene> or via <scene name='71/711660/Creb_arg_hbond/1'>hydrogen bonds</scene> with the bases. |
| + | |||
| + | Since these basic amino acids would usually repel, the <scene name='71/711660/Creb_leu/1'>leucine zipper segment</scene> allows for dimerization of the protein. Notice the spacing of the leucine residues; they are spaced by 3, 4, or 7 amino acids, causing them to be on the same face of the alpha helix. this hydrophobic set of "zipper teeth" allows this region to interact with another monomer unit, pairing with the exact same residues to form a <scene name='71/711660/Creb_hydrophobic/1'>hydrophobic core</scene>, shown in grey. At the base of the leucine zipper, where the protein meets the DNA, is positioned a <scene name='71/711660/Creb__mg/1'>magnesium ion</scene>. While you would expect a divalent cation to be surrounded by negatively charged amino acids from the protein or backbone phosphates from the DNA, its inner chelation sphere is completely composed of <scene name='71/711660/Creb_h2o_mg/1'>water molecules</scene>. The next sphere of interactions include <scene name='71/711660/Creb_lys304/1'>two lysine residues</scene> (one from each protein chain) | ||
== Zinc fingers == | == Zinc fingers == | ||
Revision as of 04:28, 3 September 2015
DNA-Protein interactions
| |||||||||||
References
This text shows how to insert references: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644