Quinone reductase
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
{{Clear}} | {{Clear}} | ||
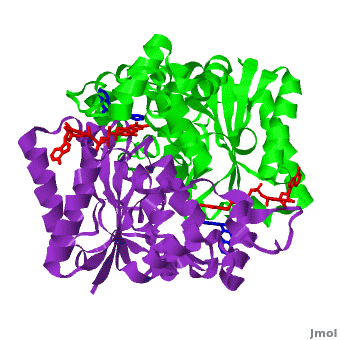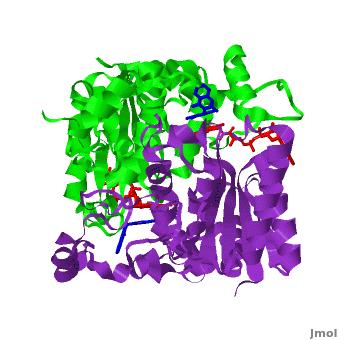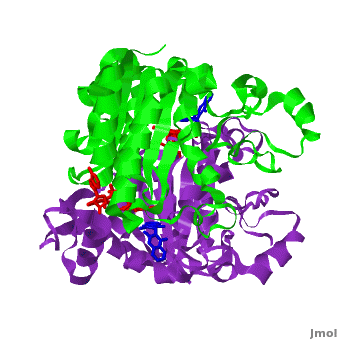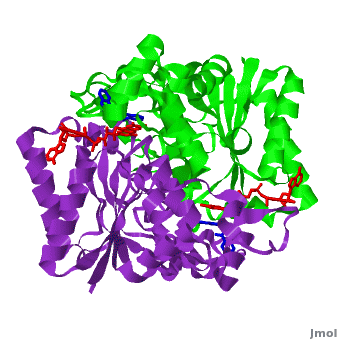
| - | <scene name='2f1o/Align/8'>Structural comparison</scene> of the active site of <font color='magenta'><b>dicoumarol/hNQO1 complex</b></font> (residues important for ligand interactions are <font color='magenta'><b>colored magenta</b></font>) with that of <font color='blue'><b>apo hNQO1</b></font> dimer ([[1d4a]], residues important for ligand interactions are <font color='blue'><b>colored blue</b></font>) reveals that structural changes associated with dicoumarol binding occur on several residues involving both monomers. < | + | <scene name='2f1o/Align/8'>Structural comparison</scene> of the active site of <font color='magenta'><b>dicoumarol/hNQO1 complex</b></font> (residues important for ligand interactions are <font color='magenta'><b>colored magenta</b></font>) with that of <font color='blue'><b>apo hNQO1</b></font> dimer ([[1d4a]], residues important for ligand interactions are <font color='blue'><b>colored blue</b></font>) reveals that structural changes associated with dicoumarol binding occur on several residues involving both monomers. <span style="color:cyan;background-color:black;font-weight:bold;">Dicoumarol is colored cyan</span>; <span style="color:orange;background-color:black;font-weight:bold;">FAD in orange</span>. The RMSD between the apo hNQO1 ([[1d4a]]) and hNQO1 in complex with dicoumarol is 0.36Å for the 546 Cα atoms. The dicoumarol-hNQO1 binding causes several structural changes. The most prominent of them is Tyr 128 and Phe 232 movement in the first monomer. These residues are located on the surface of the NQO1 catalytic pocket. The <scene name='2f1o/Align/9'>distance</scene> between these residues increases from ~5 Å in the <font color='blue'><b>apo hNQO1</b></font> to ~12 Å in the <font color='magenta'><b>dicoumarol/hNQO1 complex</b></font>. |
| - | Quinones (including duroquinone (2,3,5,6-tetramethyl-''p''-benzoquinone) are substrates of NQO1 (it catalyzes two-electron reduction of them to hydroquinones). | + | Quinones (including duroquinone (2,3,5,6-tetramethyl-''p''-benzoquinone) are substrates of NQO1 (it catalyzes two-electron reduction of them to hydroquinones). <span style="color:yellow;background-color:black;font-weight:bold;">Duroquinone (yellow)</span> binds to the <scene name='2f1o/Align1/4'>active site</scene> by interactions involving the FAD and several hydrophobic and hydrophilic residues in the duroquinone-NQO1 complex ([[1dxo]]). The structure of the hNQO1 dimer in complex with duroquinone is also similar to that of hNQO1 in complex with dicoumarol (RMSD is 0.33Å for the 546 Cα atoms). In this case, the main differences between these two structures, as well as to that of apo hNQO1, involve the distance between residues <scene name='2f1o/Align1/5'>Tyr 128 and Phe 232</scene> of the first monomer. The FAD molecule has very similar conformation in both hNQO1-duroquinone <font color='pink'><b>(pink)</b></font> and hNQO1−dicoumarol <font color='orange'><b>(orange)</b></font> complexes. Based on the comparison of NQO1 structure in complex with different NQO1 inhibitors and our previous analysis of NQO1 mutations that affect NQO1 interactions we propose that the specific conformation of Tyr 128 and Phe 232 is important for NQO1 interaction with p53 and other client proteins. |
{{Clear}} | {{Clear}} | ||
Revision as of 10:31, 9 September 2015
| |||||||||||
3D Structures of Quinone reductase
Updated on 09-September-2015
References
- Faig M, Bianchet MA, Talalay P, Chen S, Winski S, Ross D, Amzel LM. Structures of recombinant human and mouse NAD(P)H:quinone oxidoreductases: species comparison and structural changes with substrate binding and release. Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3177-82. PMID:10706635 doi:http://dx.doi.org/10.1073/pnas.050585797
- Asher G, Dym O, Tsvetkov P, Adler J, Shaul Y. The crystal structure of NAD(P)H quinone oxidoreductase 1 in complex with its potent inhibitor dicoumarol. Biochemistry. 2006 May 23;45(20):6372-8. PMID:16700548 doi:10.1021/bi0600087