Delta opioid receptor
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Ligands == | == Ligands == | ||
| - | Although opioid receptor agonists have been extremely popular throughout history, the demand for antagonists is rising. Antagonists are typically now found to be effective in treating recovery from opiate addiction as well as treatment for other psychological conditions. <scene name='71/715422/Sceneligand/1'>Naltrindole</scene> is an antagonist designed specifically for the delta opioid receptor and is a non-peptide hydrophobic compound that blocks enkephalin. Although enkephalin is a neuropeptide, Naltrindole was designed as a non-peptide in order to cross the blood brain barrier, but it still resembles encephalin in structure by having a phenyl attached indole portion that is highly recognized by the receptor <ref>doi: 10.1038/nature11111</ref>. | + | Although opioid receptor agonists have been extremely popular throughout history, the demand for antagonists is rising. Antagonists are typically now found to be effective in treating recovery from opiate addiction as well as treatment for other psychological conditions. <scene name='71/715422/Sceneligand/1'>Naltrindole</scene>, shown in crimson, is an antagonist designed specifically for the delta opioid receptor and is a non-peptide hydrophobic compound that blocks enkephalin. Although enkephalin is a neuropeptide, Naltrindole was designed as a non-peptide in order to cross the blood brain barrier, but it still resembles encephalin in structure by having a phenyl attached indole portion that is highly recognized by the receptor <ref>doi: 10.1038/nature11111</ref>. |
| - | + | ||
== Mechanism of Action == | == Mechanism of Action == | ||
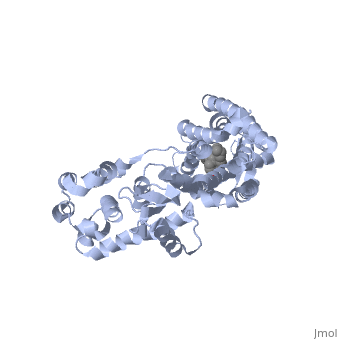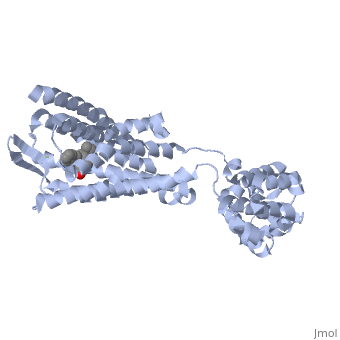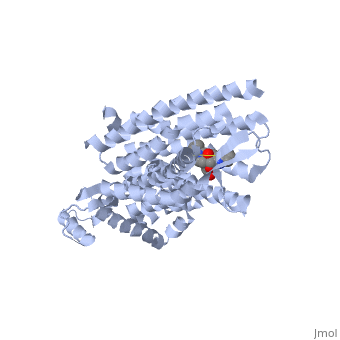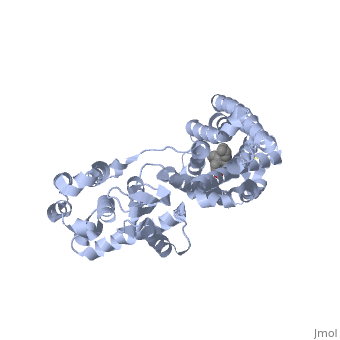
| - | Opioid receptors typically have two big portions: the upper portion that is ligand specific and recognizes a particular ligand, and the lower portion which is highly conserved amongst all receptors <ref>doi: 10.1038/nature11111</ref>. When Naltrindole approaches delta opioid receptor, it is distinguished by the high hydrophobic interaction between the indole group on the ligand and leucine 300 on the receptor. As it glides deeper into the binding site facilitated by the hydrophobic interaction, the hydroxyl group of the tyrosine-like phenol group hydrogen bonds with water molecules which are hydrogen bound to a critical histidine 248. This holds the ligand by having both the phenol group and histidine anchored by a water molecule. The water molecules within the binding pocket flank both the ligand and receptor, serving almost as a scaffolding on which for both components to act. Adjacent to the phenol group, the oxygen of an ether is hydrogen bound to tyrosine 129 of the receptor. On the opposite side of the binding site, aspartic acid 128 forms a salt bridge with the charged amino group on the ligand. The rest of the ligand maintains hydrophobic contact with non-polar residues of the binding site. The phenol to water interaction is a conserved interaction between many opioid receptors and their respective ligands as evidenced by many natural antagonists having a tyrosine that interacts with a water molecule in a similar fashion <ref>doi: 10.1038/nature11111</ref>. | + | Opioid receptors typically have two big portions: the upper portion, zoomed in here with <scene name='71/715422/Sceneactivesite/1'>active site</scene> shown in indigo, that is ligand specific and recognizes a particular ligand, and the lower portion which is highly conserved amongst all receptors <ref>doi: 10.1038/nature11111</ref>. When Naltrindole approaches delta opioid receptor, it is distinguished by the high hydrophobic interaction between the indole group on the ligand and leucine 300 on the receptor. As it glides deeper into the binding site facilitated by the hydrophobic interaction, the hydroxyl group of the tyrosine-like phenol group hydrogen bonds with water molecules which are hydrogen bound to a critical histidine 248. This holds the ligand by having both the phenol group and histidine anchored by a water molecule. The water molecules within the binding pocket flank both the ligand and receptor, serving almost as a scaffolding on which for both components to act. Adjacent to the phenol group, the oxygen of an ether is hydrogen bound to tyrosine 129 of the receptor. On the opposite side of the binding site, aspartic acid 128 forms a salt bridge with the charged amino group on the ligand. The rest of the ligand maintains hydrophobic contact with non-polar residues of the binding site. The phenol to water interaction is a conserved interaction between many opioid receptors and their respective ligands as evidenced by many natural antagonists having a tyrosine that interacts with a water molecule in a similar fashion <ref>doi: 10.1038/nature11111</ref>. |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 23:18, 9 October 2015
Background Information
| |||||||||||
References
- ↑ Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011 Dec;115(6):1363-81. doi: 10.1097/ALN.0b013e318238bba6. PMID:22020140 doi:http://dx.doi.org/10.1097/ALN.0b013e318238bba6
- ↑ Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. Structure of the delta-opioid receptor bound to naltrindole. Nature. 2012 May 16;485(7398):400-4. doi: 10.1038/nature11111. PMID:22596164 doi:10.1038/nature11111
- ↑ Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. Structure of the delta-opioid receptor bound to naltrindole. Nature. 2012 May 16;485(7398):400-4. doi: 10.1038/nature11111. PMID:22596164 doi:10.1038/nature11111
- ↑ Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. Structure of the delta-opioid receptor bound to naltrindole. Nature. 2012 May 16;485(7398):400-4. doi: 10.1038/nature11111. PMID:22596164 doi:10.1038/nature11111