Triose Phosphate Isomerase
From Proteopedia
(Difference between revisions)
| Line 49: | Line 49: | ||
{{Clear}} | {{Clear}} | ||
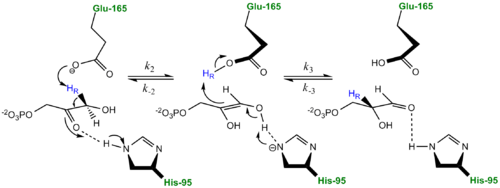
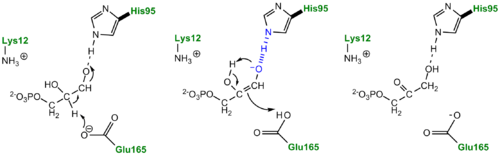
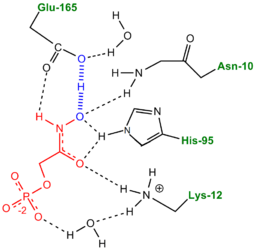
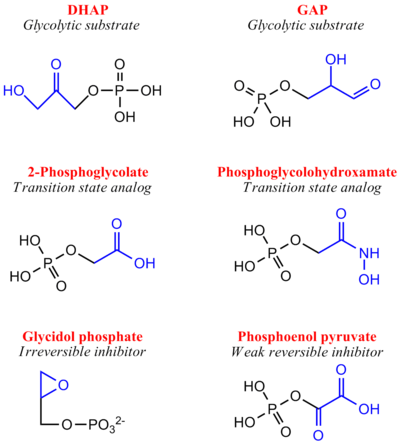
More recently a series of NMR experiments carried out by Mildvan and co-workers have shed light onto an alternative "Criss-cross" mechanism involving a LBHB between the catalytic Glu165 and the O1 oxygen of the substrate. This mechanism stipulates the His95 side chain does not directly transfer protons, this rather being accomplished entirely by Glu165. Support for this mechanism was provided by Richard and coworkers who carried out tritium labeling experiments demonstrating a significant amount of intramolecular transfer (49%) of the <sup>3</sup>H label from substrate (DHAP) to product (GAP)<ref>PMID:15709774</ref>. Using phosphoglycolohydroxamate (PGH), a mimic of the enediol(ate) intermediate, a 14.9 ppm chemical shift (6 ppm downfield) as well as a deuterium fractionation factor of 0.38 was observed with the TIM-PGH complex, corresponding to a highly deshielded proton involved in a LBHB between Glu165 and the hydroxamate oxygen of PGH. Conversely, the same NMR study found an additional hydrogen bond between the N-ε proton of His95 and the carbonyl oxygen of PGH; however, its chemical shift of 13.5 (0.4 ppm downfield from free enzyme) and fractionation factor of 0.71 indicated this was a strong H-bond, but not a LBHB.<ref>PMID:9748211</ref>. | More recently a series of NMR experiments carried out by Mildvan and co-workers have shed light onto an alternative "Criss-cross" mechanism involving a LBHB between the catalytic Glu165 and the O1 oxygen of the substrate. This mechanism stipulates the His95 side chain does not directly transfer protons, this rather being accomplished entirely by Glu165. Support for this mechanism was provided by Richard and coworkers who carried out tritium labeling experiments demonstrating a significant amount of intramolecular transfer (49%) of the <sup>3</sup>H label from substrate (DHAP) to product (GAP)<ref>PMID:15709774</ref>. Using phosphoglycolohydroxamate (PGH), a mimic of the enediol(ate) intermediate, a 14.9 ppm chemical shift (6 ppm downfield) as well as a deuterium fractionation factor of 0.38 was observed with the TIM-PGH complex, corresponding to a highly deshielded proton involved in a LBHB between Glu165 and the hydroxamate oxygen of PGH. Conversely, the same NMR study found an additional hydrogen bond between the N-ε proton of His95 and the carbonyl oxygen of PGH; however, its chemical shift of 13.5 (0.4 ppm downfield from free enzyme) and fractionation factor of 0.71 indicated this was a strong H-bond, but not a LBHB.<ref>PMID:9748211</ref>. | ||
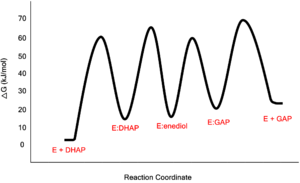
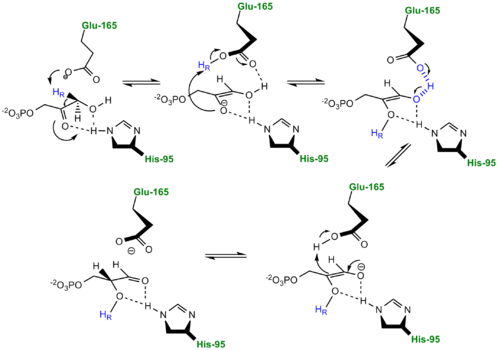
| - | [[Image: | + | [[Image:LBHB2_Glu1.png|left|thumb|500x250px|'''LBHB between Glu165 and DHAP''']] The formation of the LBHB between Glu165 and O1 of the inhibitor PGH is due to the matching of p''K''as and the alternative mechanism suggests that Glu-165, in addition to its role in initially abstracting the proton from the substrate, may also shuttle protons to and from the oxygens in the intermediate. Also, the "criss-cross" mechanism implies that, by donating a normal hydrogen bond, the role of His95 is to polarize the carbonyl oxygen and lower the p''K''a of PGH in order to facilitate subsequent proton abstraction<ref>PMID:9748211</ref>. It has been argued that that the LBHB formed between Glu165 and PGH is a consequence of using the inhibitor PGH, whose hydroxamate p''K''a of 9 better matches Glu165 then His95, and that the biological reaction would instead see the enediol forming a LHBH with His95, as mentioned above. Overall, the mechanism employed by TPI has yet to be completely solved and recent NMR studies involving both WT and mutant TPI enzymes have revealed contributions from both the "classic" and "criss-cross" mechanisms. |
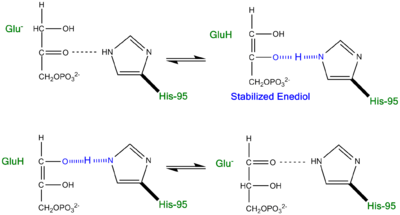
[[Image:crisscross2.png|right|thumb|750x350px| ''' Alternative "Criss-Cross" TPI Mechanism Involving LBHB Between Glu165 and O1 of the Intermediate''']] | [[Image:crisscross2.png|right|thumb|750x350px| ''' Alternative "Criss-Cross" TPI Mechanism Involving LBHB Between Glu165 and O1 of the Intermediate''']] | ||
Revision as of 13:22, 18 October 2015
| |||||||||||
Contents |
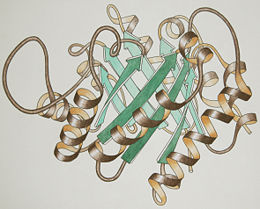
3D Structures of triose phosphate isomerase
Updated on 18-October-2015
Additional Resources
- Carbohydrate Metabolism
- Triosephosphate Isomerase by Grabowski et al.
- Triose Phosphate Isomerase Structure & Mechanism by Krenk and Reese.
Acknowledgements
The authors of this proteopedia page would like to acknowledge and thank the authors of Triosephosphate Isomerase: Paula Grabowski, Jacqueline Townsend, Kara Pryke, and Regina D. Kettering. Some content from that article was incorporated into the present article.
References
- ↑ Davenport RC, Bash PA, Seaton BA, Karplus M, Petsko GA, Ringe D. Structure of the triosephosphate isomerase-phosphoglycolohydroxamate complex: an analogue of the intermediate on the reaction pathway. Biochemistry. 1991 Jun 18;30(24):5821-6. PMID:2043623
- ↑ Harris TK, Abeygunawardana C, Mildvan AS. NMR studies of the role of hydrogen bonding in the mechanism of triosephosphate isomerase. Biochemistry. 1997 Dec 2;36(48):14661-75. PMID:9398185 doi:10.1021/bi972039v
- ↑ Saadat D, Harrison DH. The crystal structure of methylglyoxal synthase from Escherichia coli. Structure. 1999 Mar 15;7(3):309-17. PMID:10368300
- ↑ Harris TK, Abeygunawardana C, Mildvan AS. NMR studies of the role of hydrogen bonding in the mechanism of triosephosphate isomerase. Biochemistry. 1997 Dec 2;36(48):14661-75. PMID:9398185 doi:10.1021/bi972039v
- ↑ Rozovsky S, McDermott AE. Substrate product equilibrium on a reversible enzyme, triosephosphate isomerase. Proc Natl Acad Sci U S A. 2007 Feb 13;104(7):2080-5. Epub 2007 Feb 7. PMID:17287353 doi:http://dx.doi.org/0608876104
- ↑ Cleland WW, Kreevoy MM. Low-barrier hydrogen bonds and enzymic catalysis. Science. 1994 Jun 24;264(5167):1887-90. PMID:8009219
- ↑ Jogl G, Rozovsky S, McDermott AE, Tong L. Optimal alignment for enzymatic proton transfer: structure of the Michaelis complex of triosephosphate isomerase at 1.2-A resolution. Proc Natl Acad Sci U S A. 2003 Jan 7;100(1):50-5. Epub 2002 Dec 30. PMID:12509510 doi:10.1073/pnas.0233793100
- ↑ O'Donoghue AC, Amyes TL, Richard JP. Hydron transfer catalyzed by triosephosphate isomerase. Products of isomerization of (R)-glyceraldehyde 3-phosphate in D2O. Biochemistry. 2005 Feb 22;44(7):2610-21. PMID:15709774 doi:10.1021/bi047954c
- ↑ Cleland WW, Frey PA, Gerlt JA. The low barrier hydrogen bond in enzymatic catalysis. J Biol Chem. 1998 Oct 2;273(40):25529-32. PMID:9748211
- ↑ Cleland WW, Frey PA, Gerlt JA. The low barrier hydrogen bond in enzymatic catalysis. J Biol Chem. 1998 Oct 2;273(40):25529-32. PMID:9748211
- ↑ Fonvielle M, Mariano S, Therisod M. New inhibitors of rabbit muscle triose-phosphate isomerase. Bioorg Med Chem Lett. 2005 Jun 2;15(11):2906-9. PMID:15911278 doi:10.1016/j.bmcl.2005.03.061
- ↑ Fonvielle M, Mariano S, Therisod M. New inhibitors of rabbit muscle triose-phosphate isomerase. Bioorg Med Chem Lett. 2005 Jun 2;15(11):2906-9. PMID:15911278 doi:10.1016/j.bmcl.2005.03.061
- ↑ Fonvielle M, Mariano S, Therisod M. New inhibitors of rabbit muscle triose-phosphate isomerase. Bioorg Med Chem Lett. 2005 Jun 2;15(11):2906-9. PMID:15911278 doi:10.1016/j.bmcl.2005.03.061
- ↑ Kursula I, Wierenga RK. Crystal structure of triosephosphate isomerase complexed with 2-phosphoglycolate at 0.83-A resolution. J Biol Chem. 2003 Mar 14;278(11):9544-51. Epub 2003 Jan 9. PMID:12522213 doi:http://dx.doi.org/10.1074/jbc.M211389200
- ↑ Rodriguez-Almazan C, Arreola R, Rodriguez-Larrea D, Aguirre-Lopez B, de Gomez-Puyou MT, Perez-Montfort R, Costas M, Gomez-Puyou A, Torres-Larios A. Structural basis of human triosephosphate isomerase deficiency: mutation E104D is related to alterations of a conserved water network at the dimer interface. J Biol Chem. 2008 Aug 22;283(34):23254-63. Epub 2008 Jun 18. PMID:18562316 doi:10.1074/jbc.M802145200
- ↑ Schnackerz KD, Gracy RW. Probing the catalytic sites of triosephosphate isomerase by 31P-NMR with reversibly and irreversibly binding substrate analogues. Eur J Biochem. 1991 Jul 1;199(1):231-8. PMID:2065677
- ↑ http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv
- ↑ Joseph D, Petsko GA, Karplus M. Anatomy of a conformational change: hinged "lid" motion of the triosephosphate isomerase loop. Science. 1990 Sep 21;249(4975):1425-8. PMID:2402636
- ↑ Derreumaux P, Schlick T. The loop opening/closing motion of the enzyme triosephosphate isomerase. Biophys J. 1998 Jan;74(1):72-81. PMID:9449311 doi:10.1016/S0006-3495(98)77768-9
- ↑ Casteleijn MG, Alahuhta M, Groebel K, El-Sayed I, Augustyns K, Lambeir AM, Neubauer P, Wierenga RK. Functional role of the conserved active site proline of triosephosphate isomerase. Biochemistry. 2006 Dec 26;45(51):15483-94. Epub 2006 Dec 19. PMID:17176070 doi:10.1021/bi061683j
- ↑ Kursula I, Wierenga RK. Crystal structure of triosephosphate isomerase complexed with 2-phosphoglycolate at 0.83-A resolution. J Biol Chem. 2003 Mar 14;278(11):9544-51. Epub 2003 Jan 9. PMID:12522213 doi:http://dx.doi.org/10.1074/jbc.M211389200
- ↑ Schneider AS. Triosephosphate isomerase deficiency: historical perspectives and molecular aspects. Baillieres Best Pract Res Clin Haematol. 2000 Mar;13(1):119-40. PMID:10916682
- ↑ Ralser M, Heeren G, Breitenbach M, Lehrach H, Krobitsch S. Triose phosphate isomerase deficiency is caused by altered dimerization--not catalytic inactivity--of the mutant enzymes. PLoS ONE. 2006 Dec 20;1:e30. PMID:17183658 doi:10.1371/journal.pone.0000030
- ↑ Guix FX, Ill-Raga G, Bravo R, Nakaya T, de Fabritiis G, Coma M, Miscione GP, Villa-Freixa J, Suzuki T, Fernandez-Busquets X, Valverde MA, de Strooper B, Munoz FJ. Amyloid-dependent triosephosphate isomerase nitrotyrosination induces glycation and tau fibrillation. Brain. 2009 May;132(Pt 5):1335-45. Epub 2009 Feb 27. PMID:19251756 doi:10.1093/brain/awp023
- ↑ Joseph-McCarthy D, Lolis E, Komives EA, Petsko GA. Crystal structure of the K12M/G15A triosephosphate isomerase double mutant and electrostatic analysis of the active site. Biochemistry. 1994 Mar 15;33(10):2815-23. PMID:8130194
- ↑ The conservation pattern shown was calculated by ConSurfDB and might obscure some conservation due to inclusion of proteins of different functions. However in the case of 2ypi, all sequences used in the multiple sequence alignment were TPI sequences. A manual run at the ConSurf Server, using 500 TPI sequences, gave a nearly identical result. Both runs gave an average pairwise distance close to 1.0. Hence, the conservation pattern shown is correct for TPI.
Proteopedia Page Contributors and Editors (what is this?)
Gregg Snider, Eric Martz, Michal Harel, Alexander Berchansky, David Canner, Eran Hodis, Stephen Everse, Angel Herraez, Jane S. Richardson