Ivan Koutsopatriy estrogen receptor
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Function == | == Function == | ||
| - | Like other steroid hormones estrogen effects the transcription of a large number of genes via its interactions with its intracellular receptors, estrogen receptors. Estrogen receptors are receptors that are activated by the hormone estrogen (Example of an estrogen; 17β-estradiol) | + | Like other steroid hormones estrogen effects the transcription of a large number of genes via its interactions with its intracellular receptors, estrogen receptors. Estrogen receptors are receptors that are activated by the hormone estrogen (Example of an estrogen; 17β-estradiol). |
== Structural Highlights == | == Structural Highlights == | ||
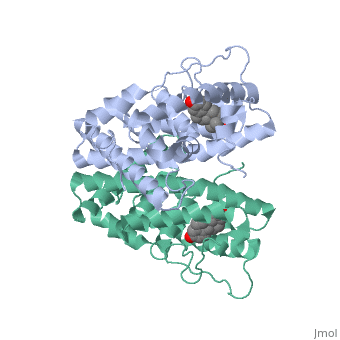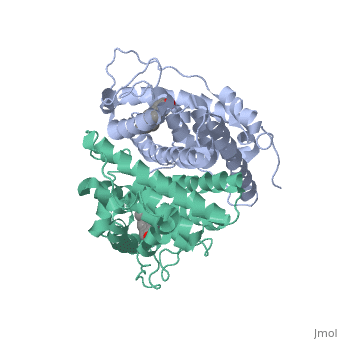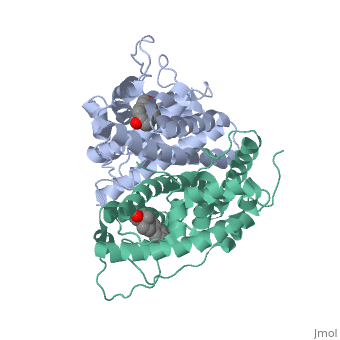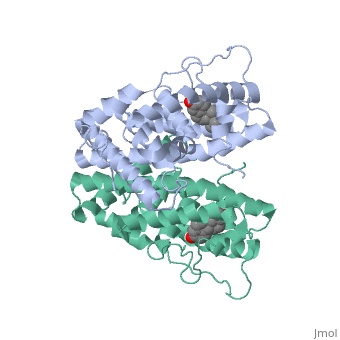
ER is a modular protein composed of a ligand binding domain, a DNA binding domain and a transactivation domain. ER is a DNA-binding transcription factor. <scene name='71/714947/Er_bound_to_dna_dnadomain/1'> ER bound to DNA with one DNA binding helix and the transactivation domain highlighted yellow </scene> The DNA binding domain can be clearly observed in this scene; the highlighted yellow helix in close proximety to the DNA is part of the DNA binding domain. The blue beta sheet close to the yellow DNA binding alpha helix is also part of the DNA binding domain. The transactivation domain is attached at the end of the yellow DNA binding domain, also forming an alpha helix colored in yellow. The transactivation domain activates RNA polymerase when the receptor binds to DNA. The ligand binding domain may be observed here with the following scene.<scene name='71/714947/Agonist_ferutinine_bound_er/5'> Agonist_ferutinine_bound_er</scene> The ligand ferutinine (highlighted in pink) is bound by the ligand binding domain, composed of the surrounding blue colored alpha helices . Unbound ER normally exists loosly around the nucleus; this is subject to change depending on a multitude of factors including cell type, progress through cell cycle and reception of cellular signals. When estrogen enters the cell and binds ER, ER trans-locates and undergoes a conformation shift.<ref> Beato, M., Chavez, S., and Truss, M. (1996). Transcriptional regulation by steroid hormones. Steroids 61: 240–251. </ref> Ligand bound estrogen receptor associates more tightly with the nucleus. | ER is a modular protein composed of a ligand binding domain, a DNA binding domain and a transactivation domain. ER is a DNA-binding transcription factor. <scene name='71/714947/Er_bound_to_dna_dnadomain/1'> ER bound to DNA with one DNA binding helix and the transactivation domain highlighted yellow </scene> The DNA binding domain can be clearly observed in this scene; the highlighted yellow helix in close proximety to the DNA is part of the DNA binding domain. The blue beta sheet close to the yellow DNA binding alpha helix is also part of the DNA binding domain. The transactivation domain is attached at the end of the yellow DNA binding domain, also forming an alpha helix colored in yellow. The transactivation domain activates RNA polymerase when the receptor binds to DNA. The ligand binding domain may be observed here with the following scene.<scene name='71/714947/Agonist_ferutinine_bound_er/5'> Agonist_ferutinine_bound_er</scene> The ligand ferutinine (highlighted in pink) is bound by the ligand binding domain, composed of the surrounding blue colored alpha helices . Unbound ER normally exists loosly around the nucleus; this is subject to change depending on a multitude of factors including cell type, progress through cell cycle and reception of cellular signals. When estrogen enters the cell and binds ER, ER trans-locates and undergoes a conformation shift.<ref> Beato, M., Chavez, S., and Truss, M. (1996). Transcriptional regulation by steroid hormones. Steroids 61: 240–251. </ref> Ligand bound estrogen receptor associates more tightly with the nucleus. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | Ferutinine also causes ER to form a tight loop allowing stimulation of normal growth. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| Line 30: | Line 21: | ||
The location of the receptor bound and unbound to ligand varies amongst different cell types. In general, an antagonist ligand will cause partial accumulation in the cytoplasm of a cell. The agonist ligand causes the translocation to the nucleus described above. This is important as ER function has been shown to vary within different phases of the cell cycle. A group bound GFP to ER and studied the location of GFP-ER upon binding of agonists and antagonist ligands. <ref> Htun H, Holth LT, Walker D, Davie JR, Hager GL (1 February 1999). "Direct visualization of the human estrogen receptor alpha reveals a role for ligand in the nuclear distribution of the receptor". Mol Biol Cell 10 (2): 471–86. </ref> GFP-ER activates the reporter gene in a dose-dependent manner and shows additional activation in the presence of agonist ligand 17-bestradiol. ICI 182780, a pure antagonist for ER, completely inhibited GFP-ER activation of the reporter gene. The group found that in the absence of ligand, the unoccupied ER is loosely associated with the nucleus and binding of a ligand causes a biochemical transformation into a complex that associates more tightly with the nucleus. | The location of the receptor bound and unbound to ligand varies amongst different cell types. In general, an antagonist ligand will cause partial accumulation in the cytoplasm of a cell. The agonist ligand causes the translocation to the nucleus described above. This is important as ER function has been shown to vary within different phases of the cell cycle. A group bound GFP to ER and studied the location of GFP-ER upon binding of agonists and antagonist ligands. <ref> Htun H, Holth LT, Walker D, Davie JR, Hager GL (1 February 1999). "Direct visualization of the human estrogen receptor alpha reveals a role for ligand in the nuclear distribution of the receptor". Mol Biol Cell 10 (2): 471–86. </ref> GFP-ER activates the reporter gene in a dose-dependent manner and shows additional activation in the presence of agonist ligand 17-bestradiol. ICI 182780, a pure antagonist for ER, completely inhibited GFP-ER activation of the reporter gene. The group found that in the absence of ligand, the unoccupied ER is loosely associated with the nucleus and binding of a ligand causes a biochemical transformation into a complex that associates more tightly with the nucleus. | ||
| + | |||
==Summary of ER DNA Interaction== | ==Summary of ER DNA Interaction== | ||
| + | |||
Before ER binds its hormone the receptor is part of a complex that has many chaperones that maintain the receptor in a steroid binding configuration. Post hormone binding the receptor dissociates from its original complex and binds to hormone responsive elements in chromatin. Gene expression is then regulated by interaction of DNA bound receptors with sequence specific transcription factors and general transcription factors which are mediated by co-activators and co-repressors. The arrangement of cis regulatory elements in a specific promoter or enhancer region and the current state of DNA sequences in nucleosomes determines the system of receptor interactions. Contingent upon the interactions occurring, the result may be induction or repression of transcription. | Before ER binds its hormone the receptor is part of a complex that has many chaperones that maintain the receptor in a steroid binding configuration. Post hormone binding the receptor dissociates from its original complex and binds to hormone responsive elements in chromatin. Gene expression is then regulated by interaction of DNA bound receptors with sequence specific transcription factors and general transcription factors which are mediated by co-activators and co-repressors. The arrangement of cis regulatory elements in a specific promoter or enhancer region and the current state of DNA sequences in nucleosomes determines the system of receptor interactions. Contingent upon the interactions occurring, the result may be induction or repression of transcription. | ||
| Line 40: | Line 33: | ||
==Conclusion== | ==Conclusion== | ||
| - | ER plays a major role in the signaling of growth and development of people. ER effects the transcription of a large number of genes | + | |
| - | + | ER plays a major role in the signaling of growth and development of people. ER effects the transcription of a large number of genes | |
| + | |||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 01:52, 30 October 2015
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Beato, M., Chavez, S., and Truss, M. (1996). Transcriptional regulation by steroid hormones. Steroids 61: 240–251.
- ↑ Wang C, Fu M, Angeletti RH, Siconolfi-Baez L, Reutens AT, Albanese C, Lisanti MP, Katzenellenbogen BS, Kato S, Hopp T, Fuqua SA, Lopez GN, Kushner PJ, Pestell RG (25 May 2001)."Direct acetylation of the estrogen receptor alpha hinge region by p300 regulates transactivation and hormone sensitivity.". J Biol Chem. 276 (21): 18375–83.
- ↑ Htun H, Holth LT, Walker D, Davie JR, Hager GL (1 February 1999). "Direct visualization of the human estrogen receptor alpha reveals a role for ligand in the nuclear distribution of the receptor". Mol Biol Cell 10 (2): 471–86.