Gunnar Reiske/Sandbox 102
From Proteopedia
m |
m |
||
| Line 24: | Line 24: | ||
=== HLA-DQ2 and HLA-DQ8 === | === HLA-DQ2 and HLA-DQ8 === | ||
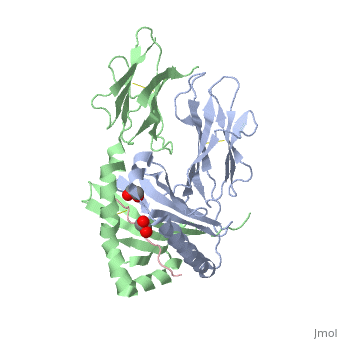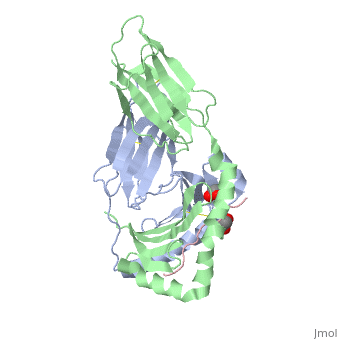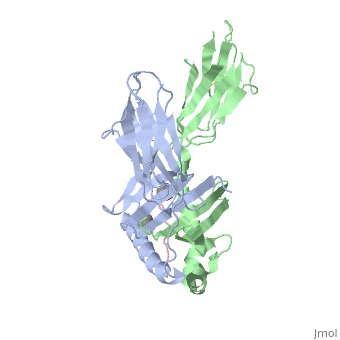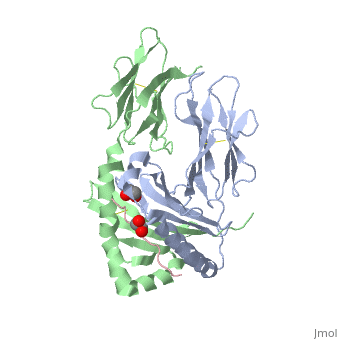
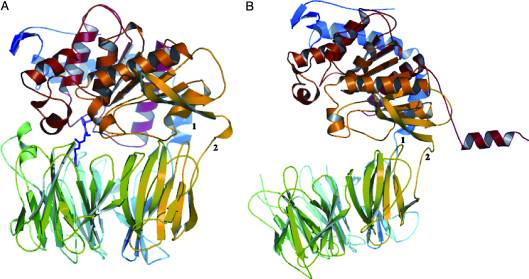
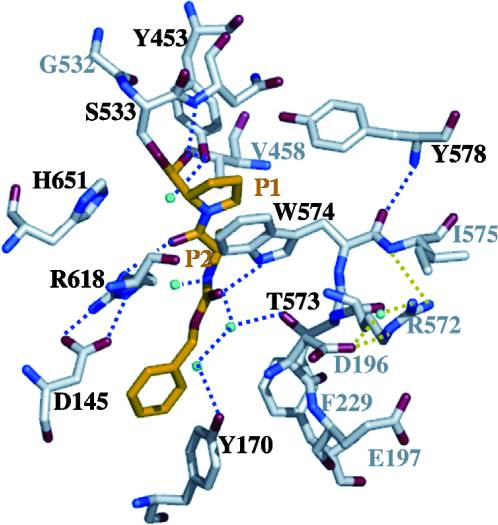
| - | HLA-DQ2 and HLA-DQ8 proteins are at high concentrations, 95% and 5 % of all celiac disease victims respectively, in those with celiac disease. The proteins are able to amplify the autoimmune response by binding the gluten complex to the transglutaminase tissue of the small intestine lumen. The new complexes are comprised of three chains, two being MHC class II antigens of alpha helical and beta sheet nature with the third being the gluten peptide. The MHC class II molecules HLA-DQ2 and HLA-DQ8 are human leukocyte antigens associated with the genetic risk of developing celiac disease and serves as a MHC class II molecule in the immune system of the body. In addition, the complexes have conformations that only expose the gliadin sequence that has gastrointestinal protease resistance. As a result, the body sends out antibodies, which bind the epitopes of the complex thus labeling it as a toxin. The end result is an amplified autoimmune response that attacks the lining of the small intestine to help rid the body of the resistant complex.<ref>Mellins, E., & Stern, L. (n.d.). HLA-DM and HLA-DO, key regulators of MHC-ll processing and presentation. Current Opinion in Immunology, 26, 115-122. February 2014. | + | HLA-DQ2 and HLA-DQ8 proteins are at high concentrations, 95% and 5% of all celiac disease victims respectively, in those with celiac disease. The proteins are able to amplify the autoimmune response by binding the gluten complex to the transglutaminase tissue of the small intestine lumen. The new complexes are comprised of three chains, two being MHC class II antigens of alpha helical and beta sheet nature with the third being the gluten peptide. The MHC class II molecules HLA-DQ2 and HLA-DQ8 are human leukocyte antigens associated with the genetic risk of developing celiac disease and serves as a MHC class II molecule in the immune system of the body. In addition, the complexes have conformations that only expose the gliadin sequence that has gastrointestinal protease resistance. As a result, the body sends out antibodies, which bind the epitopes of the complex thus labeling it as a toxin. The end result is an amplified autoimmune response that attacks the lining of the small intestine to help rid the body of the resistant complex.<ref>Mellins, E., & Stern, L. (n.d.). HLA-DM and HLA-DO, key regulators of MHC-ll processing and presentation. Current Opinion in Immunology, 26, 115-122. February 2014. |
http://www.sciencedirect.com/science/article/pii/S095279151300215X</ref> | http://www.sciencedirect.com/science/article/pii/S095279151300215X</ref> | ||
Revision as of 02:32, 16 November 2015
How Gluten Protein Structure Stimulates an Immune Response
Introduction
The protein gluten is found in wheat and grains such as rye and barley. Gluten is also involved with inducing an inflammatory response in individuals with celiac disease. Individuals who have the disease cannot digest gluten due to the protein’s structure, which will damage the small intestine. In detail, if an individual with celiac disease ingests foods containing gluten, the immune system responds by damaging the villi, which are fingerlike projections lining the small intestine. This type of immune response denies the body’s ability to absorb nutrients that pass through the small intestine and into the bloodstream. As a result of the damaged villi, people with celiac disease can become malnourished. Although celiac disease is genetic, the question of how the protein triggers an immune response in the gastrointestinal tract of affected individuals was further explored.
Gluten is a protein complex comprised of gliadin and glutenin. Gliadins, for those with celiac disease, are the principle toxic component of gluten and are composed of proline and glutamine peptide sequences. The peptides enter the circulatory system and come into contact with lymphocytes and T-cells, resulting in the release of inflammatory chemicals. The inflammatory chemicals interact with the villi of the small intestine and damage them, disabling the body from nutrient absorption. The symptoms can include abdominal pain, weight loss, fatigue, and many other symptoms associated with malnutrition. As of now, the only treatment for celiac disease is the total exclusion of gluten from the person’s diet.[1][2][3]
| |||||||||||
References
- ↑ Celiac Disease: MedlinePlus. Retrieved October 27, 2015, from https://www.nlm.nih.gov/medlineplus/celiacdisease.html
- ↑ Celiac Disease. Retrieved October 27, 2015, from http://www.niddk.nih.gov/health-information/health-topics/digestive-diseases/celiac-disease/Pages/facts.aspx
- ↑ Go to Science. Retrieved October 27, 2015, from http://www.sciencemag.org/content/297/5590/2275.full
- ↑ Maiuri, L., Ciacci, C., Ricciardelli, I., Vacca, L., Raia, V., Auricchio, S., . . . Londei, M. (2003). Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet, 362(9377), 30-37. doi:10.1016/S0140-6736(03)13803-2
- ↑ Maiuri, L., Ciacci, C., Ricciardelli, I., Vacca, L., Raia, V., Auricchio, S., . . . Londei, M. (2003). Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet, 362(9377), 30-37. doi:10.1016/S0140-6736(03)13803-2
- ↑ Mellins, E., & Stern, L. (n.d.). HLA-DM and HLA-DO, key regulators of MHC-ll processing and presentation. Current Opinion in Immunology, 26, 115-122. February 2014. http://www.sciencedirect.com/science/article/pii/S095279151300215X
- ↑ Kim, C., Quartsen, H., Bergsen, E., Khosla, C., & Sollid, L.. (n.d.). Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Cross Mark, 101(12), 4175-4179. March 2004 http://www.pnas.org/content/101/12/4175.figures-only
- ↑ Henderson, K. N., Tye-Din, J. A., Reid, H. H., Chen, Z., Borg, N. A., Beissbarth, T., . . . Anderson, R. P. (2007). A structural and immunological basis for the role of human leukocyte antigen DQ8 in celiac disease. Immunity,27(1), 23-34. doi:10.1016/j.immuni.2007.05.015
- ↑ Shan, L., I. I. Mathews, and C. Khosla. "Structural and Mechanistic Analysis of Two Prolyl nEndopeptidases: Role of Interdomain Dynamics in Catalysis and Specificity." Proceedings of the National Academy of Sciences 102.10 (2005): 3599-604. Web.
- ↑ Shan, L., I. I. Mathews, and C. Khosla. "Structural and Mechanistic Analysis of Two Prolyl nEndopeptidases: Role of Interdomain Dynamics in Catalysis and Specificity." Proceedings of the National Academy of Sciences 102.10 (2005): 3599-604. Web.
- ↑ Matysiak-Budnik, T., Candalh, C., Cellier, C., Dugave, C., Namane, A., Vidal-Martinez, T., . . . Heyman, M. (2005). Limited efficiency of prolyl-endopeptidase in the detoxification of gliadin peptides in celiac disease. Gastroenterology,129(3), 786-796. doi:10.1053/j.gastro.2005.06.016
Proteopedia Page Contributors and Editors (what is this?)
Ben Horansky, Devin Joseph, Premal Patel, Gunnar Reiske, Katlin Cannon