Disease
Smallpox is an acute, highly contagious disease which causes disfiguring and febrile rash-like illness which has no known cure. According to some health experts, smallpox was responsible for more deaths than all other infectious diseases combined thus far in the world's history. The disease caused high morbidity and mortality leading to the deaths of approximately 500 million people in the 20th century alone (Smith, 2013). This resulted in an intensive public health vaccination campaign initiated by the World Health Organization (WHO) eradicating smallpox as a human disease in the 1960’s . There were two forms of the disease worldwide: Variola major, the deadly disease, and Variola minor, a much milder form. Although naturally occurring smallpox no longer exists, there are concerns that the variola virus could be used as an agent of bioterrorism or biowarfare. As a result, it is critical to understand the molecular dynamics and virulence factors in order to prepare for a potential epidemic and to prevent the devastating consequences.
Function
The smallpox virus invades host cells by binding to specific receptors on the host cell membrane. The virus binds to host cell receptors via hemagglutinin antigens expressed on its outer surface. The exact mechanism of entry is not yet known. Upon invasion of a host cell, the smallpox virus replicates in the host cytoplasm, rather than the host nucleus, using many of it’s own enzymes to replicate. Replication begins once the virion has reached the cytoplasm of the host cell. First, a messenger RNA molecule is transcribed by RNA polymerase and related enzymes before the genome is uncoated. The first genes that are transcribed, known as early genes, code for proteins that facilitate the uncoating of the viral genome and initiate a second round of transcription of intermediate genes. The intermediate genes produce mRNA which are translated into proteins that allow the transcription of the late class of genes. The late class genes encode proteins which make up the structural and enzymatic components of a new virion. Further replication of this virus leads to the eventual shutdown of the host cell’s DNA, RNA, and protein synthesis, and causes changes to the cell’s architecture to allow the virus to use the host cell’s genetic machinery for reproduction (Berwald, 2004).
One characteristic that makes the Variola virus so deadly is that it doesn’t require the host cell’s genetic machinery to begin replication. The reason for this is that the Variola virus codes for all the enzymes needed for its proliferation. One enzyme in particular, the type IB topoisomerase is responsible for unwinding the packaged, supercoiled, viral DNA to initiate viral replication (PENN Medicine, 2006). This is accomplished through a nick-joining mechanism of the double stranded DNA, in which a tyrosine nucleophile attacks a phosphodiester bond to form a 3’ phosphotyrosine linkage and releases a free 5’ hydroxyl group. This creates a nick to unwind supercoiling, which is then re-ligated to form relaxed DNA (Perry et al, 2010). Most mechanisms involving DNA including replication, transcription, and repair induce a certain degree of positive supercoiling. Tension due to the positive supercoiling buildup can be relieved by the enzymes topoisomerase. The variola topoisomerase 1B is an unusual topoisomerase. It is by far the smallest topoisomerase (33Kda) and only acts at specific sites that contain the sequence 5’-(T/C)CCTT-3’ in the variola genome. The topoisomerase 1B is made up of two domains that wrap around this recognition sequence forming a clamp around the DNA (Minkah et al, 2007). The smaller amino terminal domain is responsible for interactions with the DNA sequence, whereas the larger C-terminal domain contains the active site and is the area responsible for all enzymatic activity.
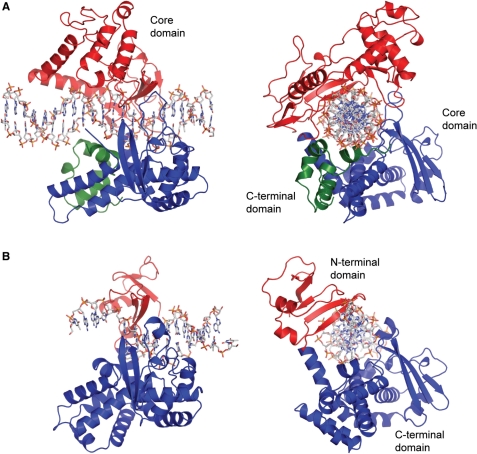
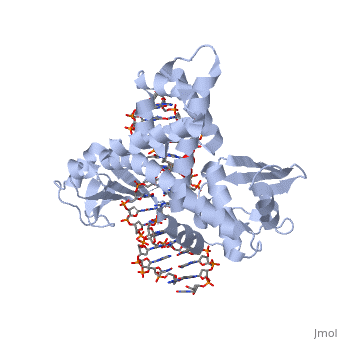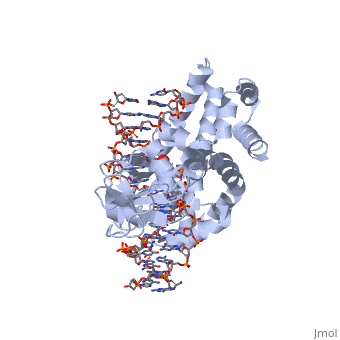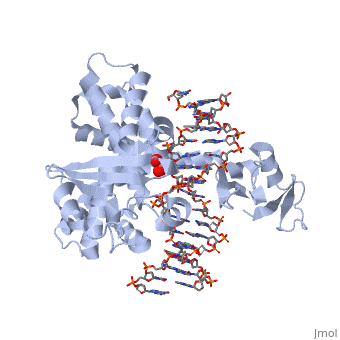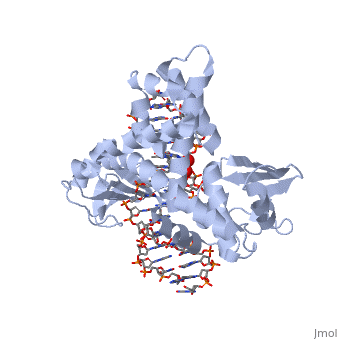
In the above figure, [A] shows the C-terminal and N-terminal domains of eukaryotic topoisomerase 1B. [B] shows the C-terminal and N-terminal domains of viral topoisomerase 1B. Both domains are highly conserved between the different species, however, the N-terminal domain of viral topoisomerase 1B is slightly smaller and more simple than the larger eukaryotic N-terminal domain. The type 1B topoisomerase can relieve negative or positive supercoiling without the use of ATP. As long as there's torsional strain on the DNA strand from the supercoiling, this is enough potential energy to drive the uncoiling of the strand. Both eukaryotic and viral topoisomerase 1B enzymes contain a highly conserved consisting of five common amino acid residues. These residues are Tyr, Arg, Arg, Lys, His/Asp.
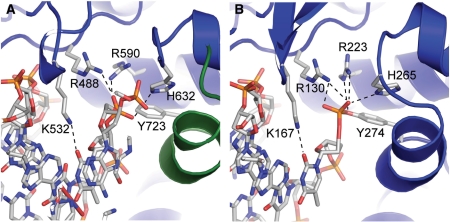
In the figure above, [A] shows the amino acid residues located within the active site of eukaryotic topoisomerase 1B. [B] shows the amino acid residues located within the active site of viral topoisomerase 1B. Both active sites contain similar residues and are highly conserved among the different species. Researchers have proven that an asparagine residue can replace the histidine residue in the active site of both eukaryotic and viral topoisomerase, and the enzyme will still undergo the same cleavage mechanism. This suggests that the same cleavage and reliagation mechanisms are the same in all topoisomerase 1B’s (Baker et al, 2009).Type IB topoisomerase is a key target for research against the spread of smallpox because it is integral for the viruses replication process. The replication of smallpox is complicated since it doesn’t hijack the host’s genetic machinery to reproduce, this makes the disease highly virulent, and hard to specifically target for elimination by antiviral drugs (Berwald, 2004).
Relevance
Smallpox is a highly contagious disease, which accounts for its massive epidemics killing millions around the world. Although smallpox was declared eradicated by the WHO in 1980, there has been recent public concern about the use of smallpox as a biological weapon. This represents a serious threat to civilian populations, which stresses the importance of understanding molecular dynamics, mechanism and, risk, in order to prevent and control it in case of an outbreak.
Structural highlights
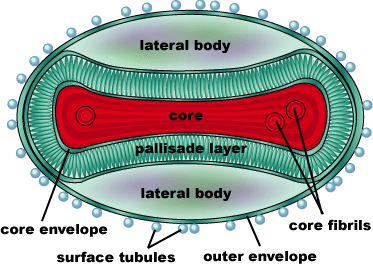
Smallpox is caused by the Variola virus, a member of the genus Orthopoxvirus (Smallpox, CDC). It’s genome consists of a single, linear double-stranded DNA molecule made up of 186,102 base pairs. There are 10 enzymes that regulate gene expression, as well as about 100 nucleoproteins involved in transcription of the DNA. The DNA and nucleoproteins are held within a biconcave (dumbbell-shaped) core with two lateral bodies on either side. The function of the lateral bodies is unknown. The outer surface of the variola virus consists of lipids and proteins that surround this core. The variola virus consists of 90% protein, 5% lipid, and 3% DNA (Shubhash and Parija, 2009).
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.