We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Bacteriorhodopsin
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Structural highlights == | == Structural highlights == | ||
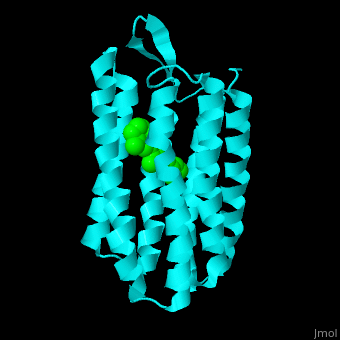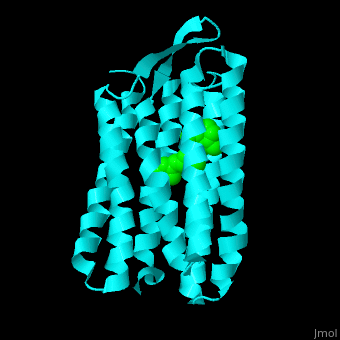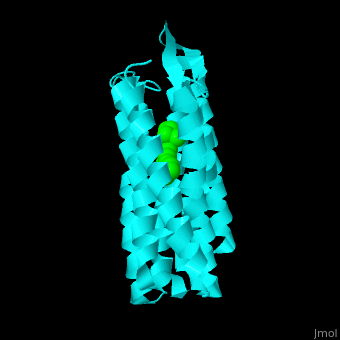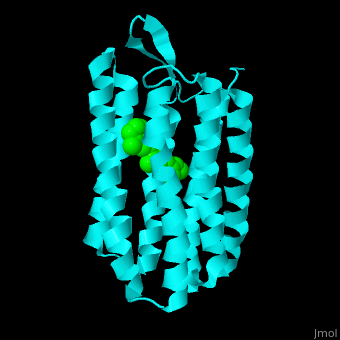
| - | Br is composed of 6 α-helical elements each containing a retinal molecule. The retinal changes its ground state conformation upon binding of a proton, causing the Br to change conformation to the activated state and pump the proton. <scene name='46/463242/Cv/3'>The retinal interacts with hydrophobic and aromatic residues</scene> | + | Br is composed of 6 α-helical elements each containing a retinal molecule. The retinal changes its ground state conformation upon binding of a proton, causing the Br to change conformation to the activated state and pump the proton. <scene name='46/463242/Cv/3'>The retinal interacts with hydrophobic and aromatic residues</scene> <ref>PMID:19603754</ref> ({{Template:ColorKey_Hydrophobic}}, {{Template:ColorKey_Polar}}). |
</StructureSection> | </StructureSection> | ||
== 3D Structures of bacteriorhodopsin == | == 3D Structures of bacteriorhodopsin == | ||
Revision as of 11:58, 30 November 2015
| |||||||||||
3D Structures of bacteriorhodopsin
Updated on 30-November-2015
See Also
References
- ↑ Joh NH, Oberai A, Yang D, Whitelegge JP, Bowie JU. Similar energetic contributions of packing in the core of membrane and water-soluble proteins. J Am Chem Soc. 2009 Aug 12;131(31):10846-7. PMID:19603754 doi:10.1021/ja904711k
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Wayne Decatur, Jaime Prilusky