Sandbox T1SS T2SS
From Proteopedia
| Line 10: | Line 10: | ||
==Structure== | ==Structure== | ||
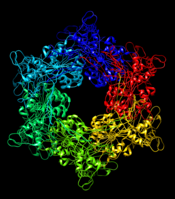
| - | [[Image:4kss.png| | + | [[Image:4kss.png|175px|left|thumb| Fig. 1. Hexametric structure of GspE (PDB: 4kss).]] |
Each T2SS is made of many general secretory proteins (GSPs). 12-15 GSPs compose one secretory system, and they are often from the same gene cluster and found on the same operon <ref name="cianciotto" />. The T2SS consists of four core components: an outer membrane complex, an inner membrane complex, an ATPase on the cytoplamsic side, and a pseudopilus. | Each T2SS is made of many general secretory proteins (GSPs). 12-15 GSPs compose one secretory system, and they are often from the same gene cluster and found on the same operon <ref name="cianciotto" />. The T2SS consists of four core components: an outer membrane complex, an inner membrane complex, an ATPase on the cytoplamsic side, and a pseudopilus. | ||
Revision as of 18:52, 9 December 2015
|
Contents |
Function
A protein is first transported from the cytoplasm to the periplasm via the Sec or Tat pathway, before being transported to the extracellular environment [1]. Proteins secreted via T2SS include proteases, cellulases, pectinases, phospholipases, lipases, and toxins, many of which are virulence factors [2]. For instance, pathogens such as Vibrio cholerae and Pseudomonas aeruginosa employ the T2SS pathway [3].
Structure
Each T2SS is made of many general secretory proteins (GSPs). 12-15 GSPs compose one secretory system, and they are often from the same gene cluster and found on the same operon [1]. The T2SS consists of four core components: an outer membrane complex, an inner membrane complex, an ATPase on the cytoplamsic side, and a pseudopilus.
The outer membrane complex consists of the GspD secretin, which uses a beta barrel structure to form a pore through the outer membrane. It is associated with the lipoprotein GspS. The inner membrane complex consists of four proteins: GspC, GspF, GspL, and GspM. Each protein has a different role in secretion, and they all interact with the protein on the periplasmic side. The ATPase is GspE. It has the Walker A motif and uses ATP hydrolysis to power the pseudopilus, which drives protein secretion. The pseudopilus is composed of GspG, GspH, GspI, GspI and GspK. There is evidence that GspG is the sole component of the stalk portion, which uses a ratcheting motion to push proteins through the membrane [1].
Energetics
In T2SS, energy for protein secretion comes from ATP. It has been suggested that GspE hydrolyzes ATP in order to drive the assembly and disassembly of the pseudopilus, which is responsible for protein transport, as well as to push proteins through the outer membrane channel [4].
References
- ↑ 1.0 1.1 1.2 Cianciotto, Nicholas. “Type II secretion: a protein secretion system for all seasons.” 2005. Trends in Microbiology 13: 581-588.
- ↑ Sandkvist, Maria. “Type II Secretion and Pathogenesis.” 2001. Infection and Immunity 69: 3523-3535.
- ↑ Lu, Connie, Stewart Turley, Samuel T. Marionni, et al. “Hexamers of the Type II Secretion ATPase GspE from Vibrio cholerae with Increased ATPase Activity.” 2013. Cell 21: 1707-1717.
- ↑ Costa, Tiago, Catarina Felisberto-Rodrigues, Amit Meir, et al. “Secretion systems in Gram-negative bacteria: structural and mechanistic insights.” 2015. Nature Reviews Microbiology 13: 343-359.