Sandbox porins
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
==Porins Overview== | ==Porins Overview== | ||
<StructureSection load='2j1n' size='340' side='right' caption='E. coli OmpC' scene=''> | <StructureSection load='2j1n' size='340' side='right' caption='E. coli OmpC' scene=''> | ||
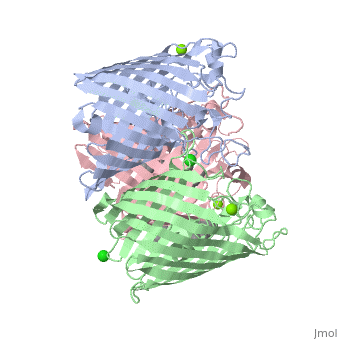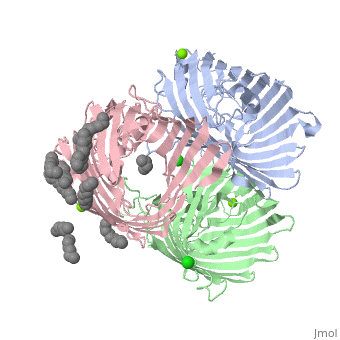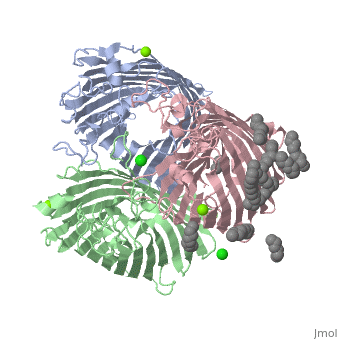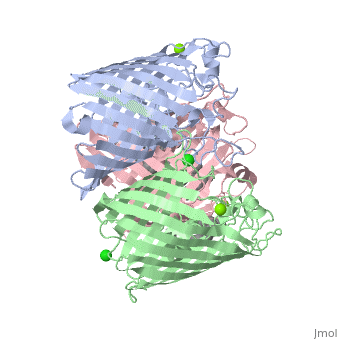
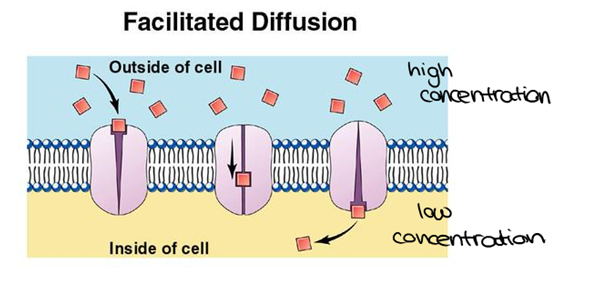
| - | Porins are proteins located in the outer membrane of gram-negative bacteria or the outer membrane of mitochondria in eukaryotes and function as simple diffusion channels. The simple diffusion channels allow diffusion of sugars, ions, and amino acids to cross the outer membrane. Formation of porins occurs when a signal sequence is used to transfer the porin protein out from the inner membrane of bacteria. For this to occur, chaperone protein Sec B containing an unfolded polypeptide chain sends out a signal. This signal is sent to Sec A ATPase and binds to the Sec B complex. The polypeptide is then released and sent to the Sec E – Sec Y translocation channel where it begins to fold into partially assembled beta-sheets. The lipid-binding region located in the hydrophobic core of the partially assembled beta-sheets will then pull the complex into the outer membrane. Once in the outer membrane, the beta-sheets completely form by the alternating polar/non-polar side chains. Once porins have formed, structural components, functions, and energetic processes can be observed. | + | Porins such as OmpC <ref> http://www.proteopedia.org/wiki/index.php/Osmoporin_OmpC_%28E._coli%29 access 12/9/15 </ref>, are proteins located in the outer membrane of gram-negative bacteria or the outer membrane of mitochondria in eukaryotes and function as simple diffusion channels. The simple diffusion channels allow diffusion of sugars, ions, and amino acids to cross the outer membrane. Formation of porins occurs when a signal sequence is used to transfer the porin protein out from the inner membrane of bacteria. For this to occur, chaperone protein Sec B containing an unfolded polypeptide chain sends out a signal. This signal is sent to Sec A ATPase and binds to the Sec B complex. The polypeptide is then released and sent to the Sec E – Sec Y translocation channel where it begins to fold into partially assembled beta-sheets. The lipid-binding region located in the hydrophobic core of the partially assembled beta-sheets will then pull the complex into the outer membrane. Once in the outer membrane, the beta-sheets completely form by the alternating polar/non-polar side chains. Once porins have formed, structural components, functions, and energetic processes can be observed. |
<ref> http://www.proteopedia.org/wiki/index.php/Osmoporin_OmpC_%28E._coli%29 access 12/9/15 </ref> | <ref> http://www.proteopedia.org/wiki/index.php/Osmoporin_OmpC_%28E._coli%29 access 12/9/15 </ref> | ||
Revision as of 18:37, 11 December 2015
This page is setup for Matt to build his senior project for OU CHEM 4923
Porins Overview
| |||||||||||
References
- ↑ http://www.proteopedia.org/wiki/index.php/Osmoporin_OmpC_%28E._coli%29 access 12/9/15
- ↑ http://www.proteopedia.org/wiki/index.php/Osmoporin_OmpC_%28E._coli%29 access 12/9/15
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
[1]
Two in-text citations from same source:
First time it's referenced: [2]
Subsequent times: Cite error: Invalid <ref> tag;
refs with no name must have content